LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer
- PMID: 38892143
- PMCID: PMC11172611
- DOI: 10.3390/ijms25115955
LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer
Abstract
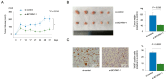
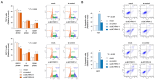
Bladder cancer (BC) is a common genitourinary malignancy that exhibits silent morbidity and high mortality rates because of a lack of diagnostic markers and limited effective treatments. Here, we evaluated the role of the lncRNA brain cytoplasmic RNA 1 (BCYRN1) in BC. We performed loss-of-function assays to examine the effects of BCYRN1 downregulation in T24 and BOY BC cells. We found that BCYRN1 downregulation significantly inhibited the proliferation, migration, invasion, and three-dimensional spheroid formation ability and induced apoptosis in BC cells. Additionally, gene set enrichment analysis (GSEA) using RNA sequences from tumor fractions showed that BCYRN1 downregulation decreased the expression of mRNAs associated with the cell cycle. These findings were supported by observations of G2/M arrest in flow cytometry assays. Finally, we examined the expression of serum exosomal BCYRN1 as a biomarker. Clinically, BCYRN1 expression in serum exosomes from patients with BC (n = 31) was significantly higher than that in healthy donors (n = 19; mean difference: 4.1-fold higher, p < 0.01). Moreover, in patients who had undergone complete resection of BC, serum exosomal BCYRN1 levels were significantly decreased (n = 8). Thus, serum exosomal BCYRN1 may be a promising diagnostic marker and therapeutic target in patients with BC.
Keywords: BCYRN1; biomarker; bladder cancer; exosome; lncRNA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Safiri S., Kolahi A.-A., Naghavi M. Global, Regional and National Burden of Bladder Cancer and Its Attributable Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. BMJ Glob. Health. 2021;6:e004128. doi: 10.1136/bmjgh-2020-004128. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

