Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice
- PMID: 38892148
- PMCID: PMC11172739
- DOI: 10.3390/ijms25115963
Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice
Abstract
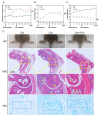
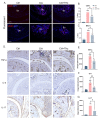
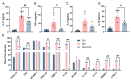
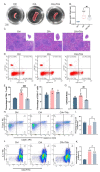
The primary emphasis of photoimmunology is the impact of nonionizing radiation on the immune system. With the development of terahertz (THz) and sub-terahertz (sub-THz) technology, the biological effects of this emerging nonionizing radiation, particularly its influence on immune function, remain insufficiently explored but are progressively attracting attention. Here, we demonstrated that 0.1 sub-THz radiation can modulate the immune system and alleviate symptoms of arthritis in collagen-induced arthritis (CIA) mice through a nonthermal manner. The application of 0.1 sub-THz irradiation led to a decrease in proinflammatory factors within the joints and serum, reducing the levels of blood immune cells and the quantity of splenic CD4+ T cells. Notably, 0.1 sub-THz irradiation restored depleted Treg cells in CIA mice and re-established the Th17/Treg equilibrium. These findings suggested that sub-THz irradiation plays a crucial role in systemic immunoregulation. Further exploration of its immune modulation mechanisms revealed the anti-inflammatory properties of 0.1 sub-THz on LPS-stimulated skin keratinocytes. Through the reduction in NF-κB signaling and NLRP3 inflammasome activation, 0.1 sub-THz irradiation effectively decreased the production of inflammatory factors and immune-active substances, including IL-1β and PGE2, in HaCaT cells. Consequently, 0.1 sub-THz irradiation mitigated the inflammatory response and contributed to the maintenance of immune tolerance in CIA mice. This research provided significant new evidence supporting the systemic impacts of 0.1 sub-THz radiation, particularly on the immune system. It also enhanced the field of photoimmunology and offered valuable insights into the potential biomedical applications of 0.1 sub-THz radiation for treating autoimmune diseases.
Keywords: CIA mice; HaCaT; immunity; inflammation; terahertz irradiation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Grundt J.E., Cerna C., Roth C.C., Ibey B.L., Lipscomb D., Echchgadda I., Wilmink G.J. Terahertz radiation triggers a signature gene expression profile in human cells; Proceedings of the 2011 International Conference on Infrared, Millimeter, and Terahertz Waves; Houston, TX, USA. 2–7 October 2011; p. 12442108.
-
- Echchgadda I., Cerna C.Z., Sloan M.A., Elam D.P., Ibey B.L. Optical Interactions with Tissue and Cells XXVI. SPIE; San Francisco, CA, USA: 2015. Effects of different terahertz frequencies on gene expression in human keratinocytes.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

