Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge
- PMID: 38892174
- PMCID: PMC11172421
- DOI: 10.3390/ijms25115986
Degradation of Toxins Derived from Foodborne Pathogens by Atmospheric-Pressure Dielectric-Barrier Discharge
Abstract
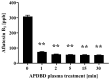
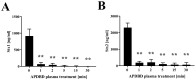
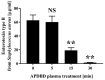
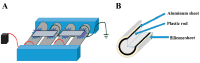
Foodborne diseases can be attributed not only to contamination with bacterial or fungal pathogens but also their associated toxins. Thus, to maintain food safety, innovative decontamination techniques for toxins are required. We previously demonstrated that an atmospheric-pressure dielectric-barrier discharge (APDBD) plasma generated by a roller conveyer plasma device is effective at inactivating bacteria and fungi in foods. Here, we have further examined whether the roller conveyer plasma device can be used to degrade toxins produced by foodborne bacterial pathogens, including aflatoxin, Shiga toxins (Stx1 and Stx2), enterotoxin B and cereulide. Each toxin was spotted onto an aluminum plate, allowed to dry, and then treated with APDBD plasma applied by the roller conveyer plasma device for different time periods. Assessments were conducted using a competitive enzyme-linked immunosorbent assay (ELISA) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). The results demonstrate a significant time-dependent decrease in the levels of these toxins. ELISA showed that aflatoxin B1 concentrations were reduced from 308.6 µg/mL to 74.4 µg/mL within 1 min. For Shiga toxins, Stx1 decreased from 913.8 µg/mL to 65.1 µg/mL, and Stx2 from 2309.0 µg/mL to 187.6 µg/mL within the same time frame (1 min). Enterotoxin B levels dropped from 62.67 µg/mL to 1.74 µg/mL at 15 min, and 1.43 µg/mL at 30 min, but did not display a significant decrease within 5 min. LC-MS/MS analysis verified that cereulide was reduced to below the detection limit following 30 min of APDBD plasma treatment. Taken together, these findings highlight that a range of foodborne toxins can be degraded by a relatively short exposure to plasma generated by an APDBD using a roller conveyer device. This technology offers promising advancements in food safety, providing a novel method to alleviate toxin contamination in the food processing industry.
Keywords: Shiga toxin; aflatoxin; bacterial toxin; cereulide; discharge; disinfection; enterotoxin; gas plasma; mycotoxin; roller conveyer; sterilization; verotoxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- World Health Organization Food Safety—Key Facts. 2022. [(accessed on 15 March 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety.
-
- International Agency for Research on Cancer . Aflatoxins, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 100F. World Health Organization; Lyon, France: 2012. [(accessed on 15 March 2024)]. Available online: https://publications.iarc.fr/123.
-
- Centers for Disease Control and Prevention E. coli and Food Safety. [(accessed on 15 March 2024)];2024 Available online: https://www.cdc.gov/foodsafety/communication/ecoli-and-food-safety.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

