CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy
- PMID: 38892190
- PMCID: PMC11172745
- DOI: 10.3390/ijms25116003
CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy
Abstract
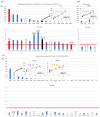
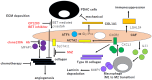
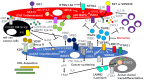
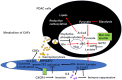
Pancreatic ductal adenocarcinoma (PDAC) is the most aggressive cancer with striking fibrosis, and its mortality rate is ranked second across human cancers. Cancer-associated fibroblasts (CAFs) play a critical role in PDAC progression, and we reviewed the molecular understanding of PDAC CAFs and novel therapeutic potential at present. CAFs-associated genes (CAFGs) were tentatively classified into three categories by stroma specificity representing stroma/epithelia expression ratios (SE ratios). The recent classification using single cell transcriptome technology clarified that CAFs were composed of myofibroblasts (myCAFs), inflammatory CAFs (iCAFs), and other minor ones (e.g., POSTN-CAFs and antigen presenting CAFs, apCAFs). LRRC15 is a myCAFs marker, and myCAFs depletion by diphtheria toxin induces the rapid accumulation of cytotoxic T lymphocytes (CTLs) and therefore augment PDL1 antibody treatments. This finding proposes that myCAFs may be a critical regulator of tumor immunity in terms of PDAC progression. myCAFs are located in CAFs adjacent to tumor cells, while iCAFs marked by PDPN and/or COL14A1 are distant from tumor cells, where hypoxic and acidic environments being located in iCAFs putatively due to poor blood supply is consistent with HIF1A and GPR68 expressions. iCAFs may be shared with SASP (secretion-associated phenotypes) in senescent CAFs. myCAFs are classically characterized by CAFGs induced by TGFB1, while chemoresistant CAFs with SASP may dependent on IL6 expression and accompanied by STAT3 activation. Recently, it was found that the unique metabolism of CAFs can be targeted to prevent PDAC progression, where PDAC cells utilize glucose, whereas CAFs in turn utilize lactate, which may be epigenetically regulated, mediated by its target genes including CXCR4. In summary, CAFs have unique molecular characteristics, which have been rigorously clarified as novel therapeutic targets of PDAC progression.
Keywords: CAFs; LRRC15; PDAC; SPARC; iCAFs; metabolism; myCAFs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shaashua L., Ben-Shmuel A., Pevsner-Fischer M., Friedman G., Levi-Galibov O., Nandakumar S., Barki D., Nevo R., Brown L.E., Zhang W., et al. BRCA mutational status shapes the stromal microenvironment of pancreatic cancer linking clusterin expression in cancer associated fibroblasts with HSF1 signaling. Nat. Commun. 2022;13:6513. doi: 10.1038/s41467-022-34081-3. - DOI - PMC - PubMed
-
- Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

