Royal Jelly: Biological Action and Health Benefits
- PMID: 38892209
- PMCID: PMC11172503
- DOI: 10.3390/ijms25116023
Royal Jelly: Biological Action and Health Benefits
Abstract
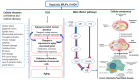
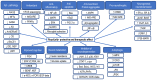
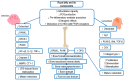
Royal jelly (RJ) is a highly nutritious natural product with great potential for use in medicine, cosmetics, and as a health-promoting food. This bee product is a mixture of important compounds, such as proteins, vitamins, lipids, minerals, hormones, neurotransmitters, flavonoids, and polyphenols, that underlie the remarkable biological and therapeutic activities of RJ. Various bioactive molecules like 10-hydroxy-2-decenoic acid (10-HDA), antibacterial protein, apisin, the major royal jelly proteins, and specific peptides such as apisimin, royalisin, royalactin, apidaecin, defensin-1, and jelleins are characteristic ingredients of RJ. RJ shows numerous physiological and pharmacological properties, including vasodilatory, hypotensive, antihypercholesterolaemic, antidiabetic, immunomodulatory, anti-inflammatory, antioxidant, anti-aging, neuroprotective, antimicrobial, estrogenic, anti-allergic, anti-osteoporotic, and anti-tumor effects. Moreover, RJ may reduce menopause symptoms and improve the health of the reproductive system, liver, and kidneys, and promote wound healing. This article provides an overview of the molecular mechanisms underlying the beneficial effects of RJ in various diseases, aging, and aging-related complications, with special emphasis on the bioactive components of RJ and their health-promoting properties. The data presented should be an incentive for future clinical studies that hopefully will advance our knowledge about the therapeutic potential of RJ and facilitate the development of novel RJ-based therapeutic opportunities for improving human health and well-being.
Keywords: aphytherapy; bioactive components; molecular and cellular activity; royal jelly.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Recent research directions on functional royal jelly: highlights prospects in food, nutraceutical, and pharmacological industries.Crit Rev Food Sci Nutr. 2024 Oct 23:1-14. doi: 10.1080/10408398.2024.2418892. Online ahead of print. Crit Rev Food Sci Nutr. 2024. PMID: 39440352 Review.
-
Health Promoting Properties of Bee Royal Jelly: Food of the Queens.Nutrients. 2021 Feb 7;13(2):543. doi: 10.3390/nu13020543. Nutrients. 2021. PMID: 33562330 Free PMC article. Review.
-
Molecular Insights into Royal Jelly Anti-Inflammatory Properties and Related Diseases.Life (Basel). 2023 Jul 17;13(7):1573. doi: 10.3390/life13071573. Life (Basel). 2023. PMID: 37511948 Free PMC article. Review.
-
Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand.Molecules. 2023 Jan 19;28(3):996. doi: 10.3390/molecules28030996. Molecules. 2023. PMID: 36770665 Free PMC article.
-
Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans.Int J Mol Sci. 2019 Sep 20;20(19):4662. doi: 10.3390/ijms20194662. Int J Mol Sci. 2019. PMID: 31547049 Free PMC article. Review.
Cited by
-
Antimicrobial Properties of Hive Products and Their Potential Applications in Human and Veterinary Medicine.Antibiotics (Basel). 2025 Feb 10;14(2):172. doi: 10.3390/antibiotics14020172. Antibiotics (Basel). 2025. PMID: 40001416 Free PMC article. Review.
-
Royal Jelly Supplementation Improves Endurance and Mitochondrial Biogenesis in Athletes: A Crossover Trial.Food Sci Nutr. 2025 Jul 16;13(7):e70497. doi: 10.1002/fsn3.70497. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40678328 Free PMC article.
-
Apitherapy with Royal Jelly and Green Propolis EPP-AF® Improves Cardiovascular Risk Markers in Patients Undergoing Hemodialysis.Toxins (Basel). 2025 Jul 26;17(8):369. doi: 10.3390/toxins17080369. Toxins (Basel). 2025. PMID: 40864045 Free PMC article. Clinical Trial.
-
The effect of royal jelly in oxidative stress, athletic performance, and mitochondrial biogenesis-related gene expression in endurance athletes: study protocol for a double-blind crossover trial.Trials. 2025 Feb 26;26(1):69. doi: 10.1186/s13063-025-08780-3. Trials. 2025. PMID: 40001097 Free PMC article.
-
Mechanistic exploration of royal jelly production in caged honey bees (Apis mellifera).Sci Rep. 2024 Dec 5;14(1):30277. doi: 10.1038/s41598-024-82094-3. Sci Rep. 2024. PMID: 39633060 Free PMC article.
References
-
- Popescu O., Mǎrghitaş L.A., Dezmirean D.S. A study about physicochemical composition of fresh and lyophilized royal jelly. Zooteh. Si Biotehnol. 2008;41:328–332.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

