Effect of Hydroxyurea on Morphology, Proliferation, and Protein Expression on Taenia crassiceps WFU Strain
- PMID: 38892261
- PMCID: PMC11172544
- DOI: 10.3390/ijms25116061
Effect of Hydroxyurea on Morphology, Proliferation, and Protein Expression on Taenia crassiceps WFU Strain
Abstract
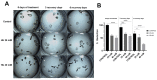
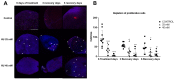
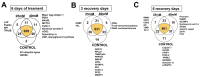
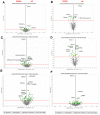
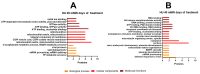
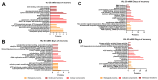
Flatworms are known for their remarkable regenerative ability, one which depends on totipotent cells known as germinative cells in cestodes. Depletion of germinative cells with hydroxyurea (HU) affects the regeneration of the parasite. Here, we studied the reduction and recovery of germinative cells in T. crassiceps cysticerci after HU treatment (25 mM and 40 mM of HU for 6 days) through in vitro assays. Viability and morphological changes were evaluated. The recovery of cysticerci's mobility and morphology was evaluated at 3 and 6 days, after 6 days of treatment. The number of proliferative cells was evaluated using EdU. Our results show morphological changes in the size, shape, and number of evaginated cysticerci at the 40 mM dose. The mobility of cysticerci was lower after 6 days of HU treatment at both concentrations. On days 3 and 6 of recovery after 25 mM of HU treatment, a partial recovery of the proliferative cells was observed. Proteomic and Gene Ontology analyses identified modifications in protein groups related to DNA binding, DNA damage, glycolytic enzymes, cytoskeleton, skeletal muscle, and RNA binding.
Keywords: Taenia crassiceps; cysticercus; hydroxyurea; proliferative cells; proteome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The synthesis of steroids by Taenia crassiceps WFU cysticerci and tapeworms is related to the developmental stages of the parasites.Gen Comp Endocrinol. 2018 Apr 1;259:154-160. doi: 10.1016/j.ygcen.2017.11.018. Epub 2017 Nov 22. Gen Comp Endocrinol. 2018. PMID: 29174867
-
Steroid synthesis by Taenia crassiceps WFU cysticerci is regulated by enzyme inhibitors.Gen Comp Endocrinol. 2013 Jul 1;188:212-7. doi: 10.1016/j.ygcen.2013.03.034. Epub 2013 Apr 20. Gen Comp Endocrinol. 2013. PMID: 23608546
-
Analysis of the expression of cytoskeletal proteins of Taenia crassiceps ORF strain cysticerci (Cestoda).Parasitol Res. 2014 May;113(5):1955-69. doi: 10.1007/s00436-014-3846-4. Epub 2014 Mar 21. Parasitol Res. 2014. PMID: 24652446
-
Steroid hormone production by parasites: the case of Taenia crassiceps and Taenia solium cysticerci.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):221-5. doi: 10.1016/s0960-0760(03)00233-4. J Steroid Biochem Mol Biol. 2003. PMID: 12943707 Review.
-
Metabolic effects of anthelminthic drugs in the larval stage of the cestode Taenia crassiceps, cysticercosis experimental model - A review.Acta Trop. 2020 Jun;206:105448. doi: 10.1016/j.actatropica.2020.105448. Epub 2020 Mar 16. Acta Trop. 2020. PMID: 32194066 Review.
References
-
- Goesseringer N., Lindenblatt N., Mihic-Probst D., Grimm F., Giovanoli P. Taenia crassiceps upper limb fasciitis in a patient with untreated acquired immunodeficiency syndrome and chronic hepatitis C infection—The role of surgical debridement. J. Plast. Reconstr. Aesthet. Surg. 2011;64:e174–e176. doi: 10.1016/j.bjps.2011.02.011. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

