Unraveling the Role of Reactive Oxygen Species in T Lymphocyte Signaling
- PMID: 38892300
- PMCID: PMC11172744
- DOI: 10.3390/ijms25116114
Unraveling the Role of Reactive Oxygen Species in T Lymphocyte Signaling
Abstract
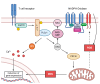
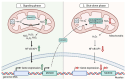
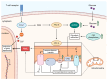
Reactive oxygen species (ROS) are central to inter- and intracellular signaling. Their localized and transient effects are due to their short half-life, especially when generated in controlled amounts. Upon T cell receptor (TCR) activation, regulated ROS signaling is primarily initiated by complexes I and III of the electron transport chain (ETC). Subsequent ROS production triggers the activation of nicotinamide adenine dinucleotide phosphate oxidase 2 (NADPH oxidase 2), prolonging the oxidative signal. This signal then engages kinase signaling cascades such as the mitogen-activated protein kinase (MAPK) pathway and increases the activity of REDOX-sensitive transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1). To limit ROS overproduction and prevent oxidative stress, nuclear factor erythroid 2-related factor 2 (Nrf2) and antioxidant proteins such as superoxide dismutases (SODs) finely regulate signal intensity and are capable of terminating the oxidative signal when needed. Thus, oxidative signals, such as T cell activation, are well-controlled and critical for cellular communication.
Keywords: T cell activation; T cell receptor (TCR); T lymphocytes; electron transport chain (ETC); glycolysis; metabolic shift; oxidative signal; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation.Free Radic Biol Med. 2013 Dec;65:789-799. doi: 10.1016/j.freeradbiomed.2013.08.005. Epub 2013 Aug 14. Free Radic Biol Med. 2013. PMID: 23954472
-
Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression.J Exp Med. 2002 Jan 7;195(1):59-70. doi: 10.1084/jem.20010659. J Exp Med. 2002. PMID: 11781366 Free PMC article.
-
Jianpi Huazhuo Tiaozhi granules reduce oxidative stress injury in macrophages by inhibiting the nicotinamide adenine dinucleotide phosphate oxidase/reactive oxygen species-nuclear transcription factor kappa B pathway.J Tradit Chin Med. 2020 Dec;40(6):922-927. doi: 10.19852/j.cnki.jtcm.2020.06.004. J Tradit Chin Med. 2020. PMID: 33258343
-
T cell receptor stimulation, reactive oxygen species, and cell signaling.Free Radic Biol Med. 2004 Oct 15;37(8):1144-51. doi: 10.1016/j.freeradbiomed.2004.05.029. Free Radic Biol Med. 2004. PMID: 15451054 Review.
-
The role of Nrf2 and PPARgamma in the improvement of oxidative stress in hypertension and cardiovascular diseases.Physiol Res. 2020 Dec 31;69(Suppl 4):S541-S553. doi: 10.33549/physiolres.934612. Physiol Res. 2020. PMID: 33656904 Free PMC article. Review.
Cited by
-
Exploring the Thioredoxin System as a Therapeutic Target in Cancer: Mechanisms and Implications.Antioxidants (Basel). 2024 Sep 4;13(9):1078. doi: 10.3390/antiox13091078. Antioxidants (Basel). 2024. PMID: 39334737 Free PMC article. Review.
-
ROS-Responsive Nanoplatforms for Targeted Tumor Immunomodulation: A Paradigm Shift in Precision Cancer Immunotherapy.Pharmaceutics. 2025 Jul 5;17(7):886. doi: 10.3390/pharmaceutics17070886. Pharmaceutics. 2025. PMID: 40733095 Free PMC article. Review.
-
Living on the Edge: ROS Homeostasis in Cancer Cells and Its Potential as a Therapeutic Target.Antioxidants (Basel). 2025 Aug 16;14(8):1002. doi: 10.3390/antiox14081002. Antioxidants (Basel). 2025. PMID: 40867898 Free PMC article. Review.
-
Aloin protects against UVB-induced apoptosis by modulating integrated signaling pathways.Front Pharmacol. 2025 Jul 11;16:1584233. doi: 10.3389/fphar.2025.1584233. eCollection 2025. Front Pharmacol. 2025. PMID: 40717965 Free PMC article.
-
Paraptosis-A Distinct Pathway to Cell Death.Int J Mol Sci. 2024 Oct 25;25(21):11478. doi: 10.3390/ijms252111478. Int J Mol Sci. 2024. PMID: 39519031 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

