Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment
- PMID: 38892307
- PMCID: PMC11173125
- DOI: 10.3390/ijms25116120
Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment
Abstract
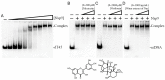
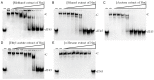
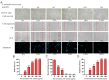
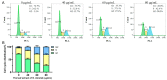
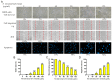
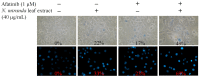
Carnivorous pitcher plants from the genus Nepenthes are renowned for their ethnobotanical uses. This research explores the therapeutic potential of Nepenthes miranda leaf extract against nonstructural protein 9 (Nsp9) of SARS-CoV-2 and in treating human non-small cell lung carcinoma (NSCLC) cell lines. Nsp9, essential for SARS-CoV-2 RNA replication, was expressed and purified, and its interaction with ssDNA was assessed. Initial tests with myricetin and oridonin, known for targeting ssDNA-binding proteins and Nsp9, respectively, did not inhibit the ssDNA-binding activity of Nsp9. Subsequent screenings of various N. miranda extracts identified those using acetone, methanol, and ethanol as particularly effective in disrupting Nsp9's ssDNA-binding activity, as evidenced by electrophoretic mobility shift assays. Molecular docking studies highlighted stigmast-5-en-3-ol and lupenone, major components in the leaf extract of N. miranda, as potential inhibitors. The cytotoxic properties of N. miranda leaf extract were examined across NSCLC lines H1975, A549, and H838, focusing on cell survival, apoptosis, and migration. Results showed a dose-dependent cytotoxic effect in the following order: H1975 > A549 > H838 cells, indicating specificity. Enhanced anticancer effects were observed when the extract was combined with afatinib, suggesting synergistic interactions. Flow cytometry indicated that N. miranda leaf extract could induce G2 cell cycle arrest in H1975 cells, potentially inhibiting cancer cell proliferation. Gas chromatography-mass spectrometry (GC-MS) enabled the tentative identification of the 19 most abundant compounds in the leaf extract of N. miranda. These outcomes underscore the dual utility of N. miranda leaf extract in potentially managing SARS-CoV-2 infection through Nsp9 inhibition and offering anticancer benefits against lung carcinoma. These results significantly broaden the potential medical applications of N. miranda leaf extract, suggesting its use not only in traditional remedies but also as a prospective treatment for pulmonary diseases. Overall, our findings position the leaf extract of N. miranda as a promising source of natural compounds for anticancer therapeutics and antiviral therapies, warranting further investigation into its molecular mechanisms and potential clinical applications.
Keywords: AntoDock; NSCLC; Nepenthes; Nsp9; SARS-CoV-2; anticancer; lupenone; plumbagin; stigmast-5-en-3-ol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Cytotoxicity and Multi-Enzyme Inhibition of Nepenthes miranda Stem Extract on H838 Human Non-Small Cell Lung Cancer Cells and RPA32, Elastase, Tyrosinase, and Hyaluronidase Proteins.Plants (Basel). 2024 Mar 11;13(6):797. doi: 10.3390/plants13060797. Plants (Basel). 2024. PMID: 38592804 Free PMC article.
-
Cytotoxic Activities and the Allantoinase Inhibitory Effect of the Leaf Extract of the Carnivorous Pitcher Plant Nepenthes miranda.Plants (Basel). 2022 Aug 31;11(17):2265. doi: 10.3390/plants11172265. Plants (Basel). 2022. PMID: 36079647 Free PMC article.
-
A natural product compound inhibits coronaviral replication in vitro by binding to the conserved Nsp9 SARS-CoV-2 protein.J Biol Chem. 2021 Dec;297(6):101362. doi: 10.1016/j.jbc.2021.101362. Epub 2021 Oct 28. J Biol Chem. 2021. PMID: 34756886 Free PMC article.
-
Coronavirus Infection-Associated Cell Death Signaling and Potential Therapeutic Targets.Molecules. 2021 Dec 9;26(24):7459. doi: 10.3390/molecules26247459. Molecules. 2021. PMID: 34946543 Free PMC article. Review.
-
Affinity selection-mass spectrometry in the discovery of anti-SARS-CoV-2 compounds.Mass Spectrom Rev. 2024 Jan-Feb;43(1):39-46. doi: 10.1002/mas.21800. Epub 2022 Aug 5. Mass Spectrom Rev. 2024. PMID: 35929396 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

