Na+/K+-ATPase: More than an Electrogenic Pump
- PMID: 38892309
- PMCID: PMC11172918
- DOI: 10.3390/ijms25116122
Na+/K+-ATPase: More than an Electrogenic Pump
Abstract
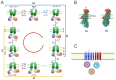
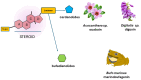
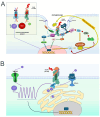
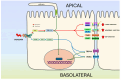
The sodium pump, or Na+/K+-ATPase (NKA), is an essential enzyme found in the plasma membrane of all animal cells. Its primary role is to transport sodium (Na+) and potassium (K+) ions across the cell membrane, using energy from ATP hydrolysis. This transport creates and maintains an electrochemical gradient, which is crucial for various cellular processes, including cell volume regulation, electrical excitability, and secondary active transport. Although the role of NKA as a pump was discovered and demonstrated several decades ago, it remains the subject of intense research. Current studies aim to delve deeper into several aspects of this molecular entity, such as describing its structure and mode of operation in atomic detail, understanding its molecular and functional diversity, and examining the consequences of its malfunction due to structural alterations. Additionally, researchers are investigating the effects of various substances that amplify or decrease its pumping activity. Beyond its role as a pump, growing evidence indicates that in various cell types, NKA also functions as a receptor for cardiac glycosides like ouabain. This receptor activity triggers the activation of various signaling pathways, producing significant morphological and physiological effects. In this report, we present the results of a comprehensive review of the most outstanding studies of the past five years. We highlight the progress made regarding this new concept of NKA and the various cardiac glycosides that influence it. Furthermore, we emphasize NKA's role in epithelial physiology, particularly its function as a receptor for cardiac glycosides that trigger intracellular signals regulating cell-cell contacts, proliferation, differentiation, and adhesion. We also analyze the role of NKA β-subunits as cell adhesion molecules in glia and epithelial cells.
Keywords: Na+/K+-ATPase; cardiac glycosides; epithelia; ion pump; receptor; signalosome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lopina O., Bukach O., Sidorenko S., Klimanova E. Na+,K+-ATPase As a Polyfunctional Protein. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2022;16:207–216. doi: 10.1134/S1990747822040055. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

