Ibulocydine Inhibits Migration and Invasion of TNBC Cells via MMP-9 Regulation
- PMID: 38892310
- PMCID: PMC11173234
- DOI: 10.3390/ijms25116123
Ibulocydine Inhibits Migration and Invasion of TNBC Cells via MMP-9 Regulation
Abstract
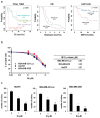
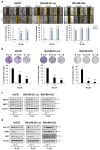
Triple-negative breast cancer (TNBC) accounts for approximately 15-20% of all breast cancer types, indicating a poor survival prognosis with a more aggressive biology of metastasis to the lung and a short response duration to available therapies. Ibulocydine (IB) is a novel (cyclin-dependent kinase) CDK7/9 inhibitor prodrug displaying potent anti-cancer effects against various cancer cell types. We performed in vitro and in vivo experiments to determine whether IB inhibits metastasis and eventually overcomes the poor drug response in TNBC. The result showed that IB inhibited the growth of TNBC cells by inducing caspase-mediated apoptosis and blocking metastasis by reducing MMP-9 expression in vitro. Concurrently, in vivo experiments using the metastasis model showed that IB inhibited metastasis of MDA-MB-231-Luc cells to the lung. Collectively, these results demonstrate that IB inhibited the growth of TNBC cells and blocked metastasis by regulating MMP-9 expression, suggesting a novel therapeutic agent for metastatic TNBC.
Keywords: ibulocydine; metastasis; triple-negative breast cancer.
Conflict of interest statement
All authors declare no competing financial or non-financial interests.
Figures



Similar articles
-
Zerumbone suppresses the motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway.Oncotarget. 2016 Jan 12;7(2):1544-58. doi: 10.18632/oncotarget.6441. Oncotarget. 2016. PMID: 26637807 Free PMC article.
-
Thymoquinone Inhibits Proliferation and Migration of MDA-MB-231 Triple Negative Breast Cancer Cells by Suppressing Autophagy, Beclin-1 and LC3.Anticancer Agents Med Chem. 2021;21(3):355-364. doi: 10.2174/1871520620666200807221047. Anticancer Agents Med Chem. 2021. PMID: 32767958
-
Berberine Suppresses Cell Motility Through Downregulation of TGF-β1 in Triple Negative Breast Cancer Cells.Cell Physiol Biochem. 2018;45(2):795-807. doi: 10.1159/000487171. Epub 2018 Jan 31. Cell Physiol Biochem. 2018. PMID: 29414799
-
MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 agonist, causes regression of orthotopic tumors and inhibits outgrowth of metastatic triple-negative breast cancer.Breast Cancer Res. 2019 Feb 18;21(1):27. doi: 10.1186/s13058-019-1116-1. Breast Cancer Res. 2019. PMID: 30777098 Free PMC article.
-
Rhizoma Amorphophalli inhibits TNBC cell proliferation, migration, invasion and metastasis through the PI3K/Akt/mTOR pathway.J Ethnopharmacol. 2018 Jan 30;211:89-100. doi: 10.1016/j.jep.2017.09.033. Epub 2017 Sep 27. J Ethnopharmacol. 2018. PMID: 28962890
Cited by
-
Molecular mechanisms underlying the inhibition of cell migration and invasion in endometriosis: Advances in pharmacological research (Review).Biomed Rep. 2025 Jul 8;23(3):152. doi: 10.3892/br.2025.2030. eCollection 2025 Sep. Biomed Rep. 2025. PMID: 40704280 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- RS-2023-00261982/Korea Health Industry Development Institute/Republic of Korea
- 2018R1D1A1B0704997013/National Research Foundation of Korea
- 2020R1F1A1073962/National Research Foundation of Korea
- 2020R1A2C2101994/National Research Foundation of Korea
- RS-2023-00227084/National Research Foundation of Korea
LinkOut - more resources
Full Text Sources
Miscellaneous

