MET Oncogene Targeting for Cancer Immunotherapy
- PMID: 38892318
- PMCID: PMC11173045
- DOI: 10.3390/ijms25116109
MET Oncogene Targeting for Cancer Immunotherapy
Abstract
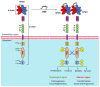
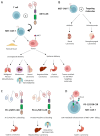
The MET receptor is one of the main drivers of 'invasive growth', a multifaceted biological response essential during embryonic development and tissue repair that is usurped by cancer cells to induce and sustain the malignant phenotype. MET stands out as one of the most important oncogenes activated in cancer and its inhibition has been explored since the initial era of cancer-targeted therapy. Different approaches have been developed to hamper MET signaling and/or reduce MET (over)expression as a hallmark of transformation. Considering the great interest gained by cancer immunotherapy, this review evaluates the opportunity of targeting MET within therapeutic approaches based on the exploitation of immune functions, either in those cases where MET impairment is crucial to induce an effective response (i.e., when MET is the driver of the malignancy), or when blocking MET represents a way for potentiating the treatment (i.e., when MET is an adjuvant of tumor fitness).
Keywords: MET antibody; MET oncogene; MET tyrosine kinase inhibitors; cellular immunotherapy; immunotherapy; targeted therapy.
Conflict of interest statement
E.V. is a co-founder of Metis Precision Medicine B-Corp (Italy). The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. A.M.L. and D.S. declare no conflicts of interest.
Figures

References
-
- Naldini L., Weidner K.M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R.P., Hartmann G., Zarnegar R., Michalopoulos G.K., et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. - DOI - PMC - PubMed
-
- Peschard P., Ishiyama N., Lin T., Lipkowitz S., Park M. A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c-Cbl/Cbl-b tyrosine kinase binding domain binding site required for suppression of oncogenic activation. J. Biol. Chem. 2004;279:29565–29571. doi: 10.1074/jbc.M403954200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

