Rhodamine 19 Alkyl Esters as Effective Antibacterial Agents
- PMID: 38892325
- PMCID: PMC11173286
- DOI: 10.3390/ijms25116137
Rhodamine 19 Alkyl Esters as Effective Antibacterial Agents
Abstract
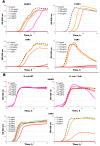
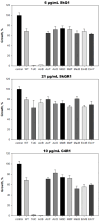
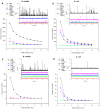
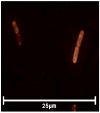
Mitochondria-targeted antioxidants (MTAs) have been studied quite intensively in recent years as potential therapeutic agents and vectors for the delivery of other active substances to mitochondria and bacteria. Their most studied representatives are MitoQ and SkQ1, with its fluorescent rhodamine analog SkQR1, a decyl ester of rhodamine 19 carrying plastoquinone. In the present work, we observed a pronounced antibacterial action of SkQR1 against Gram-positive bacteria, but virtually no effect on Gram-negative bacteria. The MDR pump AcrAB-TolC, known to expel SkQ1, did not recognize and did not pump out SkQR1 and dodecyl ester of rhodamine 19 (C12R1). Rhodamine 19 butyl (C4R1) and ethyl (C2R1) esters more effectively suppressed the growth of ΔtolC Escherichia coli, but lost their potency with the wild-type E. coli pumping them out. The mechanism of the antibacterial action of SkQR1 may differ from that of SkQ1. The rhodamine derivatives also proved to be effective antibacterial agents against various Gram-positive species, including Staphylococcus aureus and Mycobacterium smegmatis. By using fluorescence correlation spectroscopy and fluorescence microscopy, SkQR1 was shown to accumulate in the bacterial membrane. Thus, the presentation of SkQR1 as a fluorescent analogue of SkQ1 and its use for visualization should be performed with caution.
Keywords: AcrAB-TolC; MDR pumps; mitochondria-targeted antioxidants; phosphonium; rhodamine.
Conflict of interest statement
M.V.S. is the CSO of Mitotech, a company that develops new pharmaceuticals based on mitochondrially targeted antioxidants. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mitochondria-targeted antioxidants as highly effective antibiotics.Sci Rep. 2017 May 3;7(1):1394. doi: 10.1038/s41598-017-00802-8. Sci Rep. 2017. PMID: 28469140 Free PMC article.
-
An attempt to prevent senescence: a mitochondrial approach.Biochim Biophys Acta. 2009 May;1787(5):437-61. doi: 10.1016/j.bbabio.2008.12.008. Epub 2008 Dec 29. Biochim Biophys Acta. 2009. PMID: 19159610 Review.
-
Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke).Biochemistry (Mosc). 2008 Dec;73(12):1288-99. doi: 10.1134/s000629790812002x. Biochemistry (Mosc). 2008. PMID: 19120015
-
Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress.FEBS Lett. 2010 Feb 5;584(3):562-6. doi: 10.1016/j.febslet.2009.12.002. Epub 2009 Dec 6. FEBS Lett. 2010. PMID: 19995561
-
A biochemical approach to the problem of aging: "megaproject" on membrane-penetrating ions. The first results and prospects.Biochemistry (Mosc). 2007 Dec;72(12):1385-96. doi: 10.1134/s0006297907120139. Biochemistry (Mosc). 2007. PMID: 18205623 Review.
Cited by
-
Two-faced Janus SkQ1/SkQR1: patterns and molecular volume threshold for substrate recognition by the AcrAB-TolC pump.Front Pharmacol. 2025 Mar 6;16:1480955. doi: 10.3389/fphar.2025.1480955. eCollection 2025. Front Pharmacol. 2025. PMID: 40115260 Free PMC article. No abstract available.
References
-
- Antonenko Y.N., Avetisyan A.V., Cherepanov D.A., Knorre D.A., Korshunova G.A., Markova O.V., Ojovan S.M., Perevoshchikova I.V., Pustovidko A.V., Rokitskaya T.I., et al. Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers. J. Biol. Chem. 2011;286:17831–17840. doi: 10.1074/jbc.M110.212837. - DOI - PMC - PubMed
-
- Khailova L.S., Silachev D.N., Rokitskaya T.I., Avetisyan A.V., Lyamsaev K.G., Severina I.I., Il’yasova T.M., Gulyaev M.V., Dedukhova V.I., Trendeleva T.A., et al. A short-chain alkyl derivative of Rhodamine 19 acts as a mild uncoupler of mitochondria and a neuroprotector. Biochim. Biophys. Acta. 2014;1837:1739–1747. doi: 10.1016/j.bbabio.2014.07.006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

