Orthologs of NOX5 and EC-SOD/SOD3: dNox and dSod3 Impact Egg Hardening Process and Egg Laying in Reproductive Function of Drosophila melanogaster
- PMID: 38892326
- PMCID: PMC11173305
- DOI: 10.3390/ijms25116138
Orthologs of NOX5 and EC-SOD/SOD3: dNox and dSod3 Impact Egg Hardening Process and Egg Laying in Reproductive Function of Drosophila melanogaster
Abstract
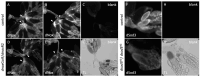
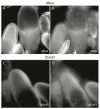
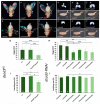
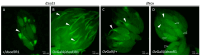
The occurrence of ovarian dysfunction is often due to the imbalance between the formation of reactive oxygen species (ROS) and the ineffectiveness of the antioxidative defense mechanisms. Primary sources of ROS are respiratory electron transfer and the activity of NADPH oxidases (NOX) while superoxide dismutases (SOD) are the main key regulators that control the levels of ROS and reactive nitrogen species intra- and extracellularly. Because of their central role SODs are the subject of research on human ovarian dysfunction but sample acquisition is low. The high degree of cellular and molecular similarity between Drosophila melanogaster ovaries and human ovaries provides this model organism with the best conditions for analyzing the role of ROS during ovarian function. In this study we clarify the localization of the ROS-producing enzyme dNox within the ovaries of Drosophila melanogaster and by a tissue-specific knockdown we show that dNox-derived ROS are involved in the chorion hardening process. Furthermore, we analyze the dSod3 localization and show that reduced activity of dSod3 impacts egg-laying behavior but not the chorion hardening process.
Keywords: (extracellular) superoxide dismutase (EC-SOD or SOD3); NADPH oxidase 5 (NOX5).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Nox regulation of smooth muscle contraction.Free Radic Biol Med. 2007 Jul 1;43(1):31-8. doi: 10.1016/j.freeradbiomed.2007.03.006. Epub 2007 Mar 12. Free Radic Biol Med. 2007. PMID: 17561091 Free PMC article.
-
NADPH oxidase-generated reactive oxygen species in mature follicles are essential for Drosophila ovulation.Proc Natl Acad Sci U S A. 2018 Jul 24;115(30):7765-7770. doi: 10.1073/pnas.1800115115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987037 Free PMC article.
-
Identification of Drosophila SOD3 and its protective role against phototoxic damage to cells.FEBS Lett. 2011 Jun 23;585(12):1973-8. doi: 10.1016/j.febslet.2011.05.033. Epub 2011 May 31. FEBS Lett. 2011. PMID: 21635891
-
Structure, regulation, and physiological functions of NADPH oxidase 5 (NOX5).J Physiol Biochem. 2023 May;79(2):383-395. doi: 10.1007/s13105-023-00955-3. Epub 2023 Mar 11. J Physiol Biochem. 2023. PMID: 36905456 Free PMC article. Review.
-
Enzymatic regulation and functional relevance of NOX5.Curr Pharm Des. 2015;21(41):5999-6008. doi: 10.2174/1381612821666151029111528. Curr Pharm Des. 2015. PMID: 26510438 Free PMC article. Review.
Cited by
-
Drosophila: A Versatile Model in Biology and Medicine.Int J Mol Sci. 2025 May 23;26(11):5032. doi: 10.3390/ijms26115032. Int J Mol Sci. 2025. PMID: 40507842 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

