Endogenous Hormone Levels and Transcriptomic Analysis Reveal the Mechanisms of Bulbil Initiation in Pinellia ternata
- PMID: 38892337
- PMCID: PMC11173086
- DOI: 10.3390/ijms25116149
Endogenous Hormone Levels and Transcriptomic Analysis Reveal the Mechanisms of Bulbil Initiation in Pinellia ternata
Abstract
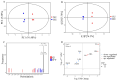
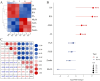
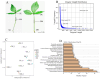
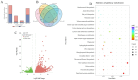
Pinellia ternata is a medicinal plant that has important pharmacological value, and the bulbils serve as the primary reproductive organ; however, the mechanisms underlying bulbil initiation remain unclear. Here, we characterized bulbil development via histological, transcriptomic, and targeted metabolomic analyses to unearth the intricate relationship between hormones, genes, and bulbil development. The results show that the bulbils initiate growth from the leaf axillary meristem (AM). In this stage, jasmonic acid (JA), abscisic acid (ABA), isopentenyl adenosine (IPA), and salicylic acid (SA) were highly enriched, while indole-3-acetic acid (IAA), zeatin, methyl jasmonate (MeJA), and 5-dexoxystrigol (5-DS) were notably decreased. Through OPLS-DA analysis, SA has emerged as the most crucial factor in initiating and positively regulating bulbil formation. Furthermore, a strong association between IPA and SA was observed during bulbil initiation. The transcriptional changes in IPT (Isopentenyltransferase), CRE1 (Cytokinin Response 1), A-ARR (Type-A Arabidopsis Response Regulator), B-ARR (Type-B Arabidopsis Response Regulator), AUX1 (Auxin Resistant 1), ARF (Auxin Response Factor), AUX/IAA (Auxin/Indole-3-acetic acid), GH3 (Gretchen Hagen 3), SAUR (Small Auxin Up RNA), GA2ox (Gibberellin 2-oxidase), GA20ox (Gibberellin 20-oxidase), AOS (Allene oxide synthase), AOC (Allene oxide cyclase), OPR (Oxophytodienoate Reductase), JMT (JA carboxy l Methyltransferase), COI1 (Coronatine Insensitive 1), JAZ (Jasmonate ZIM-domain), MYC2 (Myelocytomatosis 2), D27 (DWARF27), SMAX (Suppressor of MAX2), PAL (Phenylalanine Ammonia-Lyase), ICS (Isochorismate Synthase), NPR1 (Non-expressor of Pathogenesis-related Genes1), TGA (TGACG Sequence-specific Binding), PR-1 (Pathogenesis-related), MCSU (Molybdenium Cofactor Sulfurase), PP2C (Protein Phosphatase 2C), and SnRK (Sucrose Non-fermenting-related Protein Kinase 2) were highly correlated with hormone concentrations, indicating that bulbil initiation is coordinately controlled by multiple phytohormones. Notably, eight TFs (transcription factors) that regulate AM initiation have been identified as pivotal regulators of bulbil formation. Among these, WUS (WUSCHEL), CLV (CLAVATA), ATH1 (Arabidopsis Thaliana Homeobox Gene 1), and RAX (Regulator of Axillary meristems) have been observed to exhibit elevated expression levels. Conversely, LEAFY demonstrated contrasting expression patterns. The intricate expression profiles of these TFs are closely associated with the upregulated expression of KNOX(KNOTTED-like homeobox), suggesting a intricate regulatory network underlying the complex process of bulbil initiation. This study offers a profound understanding of the bulbil initiation process and could potentially aid in refining molecular breeding techniques specific to P. ternata.
Keywords: Pinellia ternata; bulbil; hormone; metabolomics; transcriptomics.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Transcriptomic and biochemical insights into key gene networks driving bulbil development of Pinellia ternata (Thunb.) Breit.PLoS One. 2025 Feb 11;20(2):e0314396. doi: 10.1371/journal.pone.0314396. eCollection 2025. PLoS One. 2025. PMID: 39932947 Free PMC article.
-
Effects of different hormones on the color of tree peony leaves.BMC Plant Biol. 2025 Jul 2;25(1):822. doi: 10.1186/s12870-025-06837-8. BMC Plant Biol. 2025. PMID: 40604434 Free PMC article.
-
mRNA-Seq and miRNA-Seq Analyses Provide Insights into the Mechanism of Pinellia ternata Bulbil Initiation Induced by Phytohormones.Genes (Basel). 2023 Aug 29;14(9):1727. doi: 10.3390/genes14091727. Genes (Basel). 2023. PMID: 37761867 Free PMC article.
-
A review of the interaction mechanisms between jasmonic acid (JA) and various plant hormones, as well as the core regulatory role of MYC2.Plant Sci. 2025 Apr;353:112407. doi: 10.1016/j.plantsci.2025.112407. Epub 2025 Jan 31. Plant Sci. 2025. PMID: 39894056 Review.
-
Four shades of detachment: regulation of floral organ abscission.Plant Signal Behav. 2014;9(11):e976154. doi: 10.4161/15592324.2014.976154. Plant Signal Behav. 2014. PMID: 25482787 Free PMC article. Review.
Cited by
-
Transcriptomic and biochemical insights into key gene networks driving bulbil development of Pinellia ternata (Thunb.) Breit.PLoS One. 2025 Feb 11;20(2):e0314396. doi: 10.1371/journal.pone.0314396. eCollection 2025. PLoS One. 2025. PMID: 39932947 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

