Rapid Determination of SARS-CoV-2 Integrity and Infectivity by Using Propidium Monoazide Coupled with Digital Droplet PCR
- PMID: 38892344
- PMCID: PMC11172733
- DOI: 10.3390/ijms25116156
Rapid Determination of SARS-CoV-2 Integrity and Infectivity by Using Propidium Monoazide Coupled with Digital Droplet PCR
Abstract
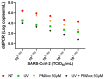
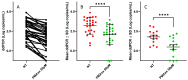
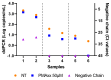
SARS-CoV-2 is a highly infectious virus responsible for the COVID-19 pandemic. Therefore, it is important to assess the risk of SARS-CoV-2 infection, especially in persistently positive patients. Rapid discrimination between infectious and non-infectious viruses aids in determining whether prevention, control, and treatment measures are necessary. For this purpose, a method was developed and utilized involving a pre-treatment with 50 µM of propidium monoazide (PMAxx, a DNA intercalant) combined with a digital droplet PCR (ddPCR). The ddPCR method was performed on 40 nasopharyngeal swabs (NPSs) both before and after treatment with PMAxx, revealing a reduction in the viral load at a mean of 0.9 Log copies/mL (SD ± 0.6 Log copies/mL). Furthermore, six samples were stratified based on the Ct values of SARS-CoV-2 RNA (Ct < 20, 20 < Ct < 30, Ct > 30) and analyzed to compare the results obtained via a ddPCR with viral isolation and a negative-chain PCR. Of the five samples found positive via a ddPCR after the PMAxx treatment, two of the samples showed the highest post-treatment SARS-CoV-2 loads. The virus was isolated in vitro from both samples and the negative strand chains were detected. In three NPS samples, SARS CoV-2 was present post-treatment at a low level; it was not isolated in vitro, and, when detected, the strand was negative. Our results indicate that the established method is useful for determining whether the SARS-CoV-2 within positive NPS samples is intact and capable of causing infection.
Keywords: PMA; PMAxx; SARS-CoV-2; ddPCR; digital droplet PCR; negative-chain PCR; propidium monoazide; viral isolation; virus infectivity; virus integrity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Measurement of the Infection and Integrity of Monkeypox Virus: A New Method Using PMAxx-ddPCR.Int J Mol Sci. 2025 Jan 30;26(3):1195. doi: 10.3390/ijms26031195. Int J Mol Sci. 2025. PMID: 39940961 Free PMC article.
-
Advancing COVID-19 diagnostics: rapid detection of intact SARS-CoV-2 using viability RT-PCR assay.Microbiol Spectr. 2024 Sep 3;12(9):e0016024. doi: 10.1128/spectrum.00160-24. Epub 2024 Jul 22. Microbiol Spectr. 2024. PMID: 39037224 Free PMC article.
-
Validation of a One-Step Reverse Transcription-Droplet Digital PCR (RT-ddPCR) Approach to Detect and Quantify SARS-CoV-2 RNA in Nasopharyngeal Swabs.Dis Markers. 2021 Mar 2;2021:8890221. doi: 10.1155/2021/8890221. eCollection 2021. Dis Markers. 2021. PMID: 33747257 Free PMC article.
-
Analytical and Clinical Performance of Droplet Digital PCR in the Detection and Quantification of SARS-CoV-2.Mol Diagn Ther. 2021 Sep;25(5):617-628. doi: 10.1007/s40291-021-00547-1. Epub 2021 Jul 28. Mol Diagn Ther. 2021. PMID: 34319580 Free PMC article.
-
In pursuit of sensitivity: Lessons learned from viral nucleic acid detection and quantification on the Raindance ddPCR platform.Methods. 2022 May;201:82-95. doi: 10.1016/j.ymeth.2021.04.008. Epub 2021 Apr 9. Methods. 2022. PMID: 33839286 Free PMC article. Review.
Cited by
-
Evaluation of Torquetenovirus (TTV) Particle Integrity Utilizing PMAxx™.Int J Mol Sci. 2025 Jul 7;26(13):6542. doi: 10.3390/ijms26136542. Int J Mol Sci. 2025. PMID: 40650319 Free PMC article.
-
Measurement of the Infection and Integrity of Monkeypox Virus: A New Method Using PMAxx-ddPCR.Int J Mol Sci. 2025 Jan 30;26(3):1195. doi: 10.3390/ijms26031195. Int J Mol Sci. 2025. PMID: 39940961 Free PMC article.
References
-
- World Health Organization. [(accessed on 3 May 2024)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- Chan M., Linn M.M.N., O’Hagan T., Guerra-Assunção J.A., Lackenby A., Workman S., Dacre A., Burns S.O., Breuer J., Hart J., et al. Persistent SARS-CoV-2 PCR Positivity Despite Anti-Viral Treatment in Immunodeficient Patients. J. Clin. Immunol. 2023;43:1083–1092. doi: 10.1007/s10875-023-01504-9. - DOI - PMC - PubMed
-
- Grimaldi P., Russo A., Pisaturo M., Maggi P., Allegorico E., Gentile I., Sangiovanni V., Rossomando A., Pacilio R., Calabria G., et al. Clinical and epidemiological factors causing longer SARS-CoV-2 viral shedding: The results from the CoviCamp cohort. Infection. 2024;52:439–446. doi: 10.1007/s15010-023-02095-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

