Inflammasomes Are Influenced by Epigenetic and Autophagy Mechanisms in Colorectal Cancer Signaling
- PMID: 38892354
- PMCID: PMC11173330
- DOI: 10.3390/ijms25116167
Inflammasomes Are Influenced by Epigenetic and Autophagy Mechanisms in Colorectal Cancer Signaling
Abstract
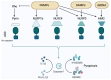
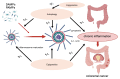
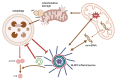
Inflammasomes contribute to colorectal cancer signaling by primarily inducing inflammation in the surrounding tumor microenvironment. Its role in inflammation is receiving increasing attention, as inflammation has a protumor effect in addition to inducing tissue damage. The inflammasome's function is complex and controlled by several layers of regulation. Epigenetic processes impact the functioning or manifestation of genes that are involved in the control of inflammasomes or the subsequent signaling cascades. Researchers have intensively studied the significance of epigenetic mechanisms in regulation, as they encompass several potential therapeutic targets. The regulatory interactions between the inflammasome and autophagy are intricate, exhibiting both advantageous and harmful consequences. The regulatory aspects between the two entities also encompass several therapeutic targets. The relationship between the activation of the inflammasome, autophagy, and epigenetic alterations in CRC is complex and involves several interrelated pathways. This article provides a brief summary of the newest studies on how epigenetics and autophagy control the inflammasome, with a special focus on their role in colorectal cancer. Based on the latest findings, we also provide an overview of the latest therapeutic ideas for this complex network.
Keywords: autophagy; colitis; colorectal cancer; epigenetics; inflammasome; regulation; therapeutic target; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Inflammasomes in cancer: Effect of epigenetic and autophagic modulations.Semin Cancer Biol. 2022 Aug;83:399-412. doi: 10.1016/j.semcancer.2020.09.013. Epub 2020 Oct 9. Semin Cancer Biol. 2022. PMID: 33039557 Review.
-
Inflammasome signaling in colorectal cancer.Transl Res. 2023 Feb;252:45-52. doi: 10.1016/j.trsl.2022.09.002. Epub 2022 Sep 21. Transl Res. 2023. PMID: 36150688 Free PMC article. Review.
-
Epigenetic modulation of autophagy pathway by small molecules in colorectal cancer: a systematic review.J Cancer Res Clin Oncol. 2024 Oct 23;150(10):474. doi: 10.1007/s00432-024-05982-1. J Cancer Res Clin Oncol. 2024. PMID: 39441422 Free PMC article.
-
NLRP3 Inflammasome: A Possible Link Between Obesity-Associated Low-Grade Chronic Inflammation and Colorectal Cancer Development.Front Immunol. 2018 Dec 11;9:2918. doi: 10.3389/fimmu.2018.02918. eCollection 2018. Front Immunol. 2018. PMID: 30619282 Free PMC article. Review.
-
Epigenetic regulation of autophagy by non-coding RNAs and exosomal non-coding RNAs in colorectal cancer: A narrative review.Int J Biol Macromol. 2024 Jul;273(Pt 2):132732. doi: 10.1016/j.ijbiomac.2024.132732. Epub 2024 May 31. Int J Biol Macromol. 2024. PMID: 38823748 Review.
Cited by
-
Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer.Int J Mol Sci. 2024 Oct 18;25(20):11201. doi: 10.3390/ijms252011201. Int J Mol Sci. 2024. PMID: 39456981 Free PMC article. Review.
-
An overview of potential of natural compounds to regulate epigenetic modifications in colorectal cancer: a recent update.Epigenetics. 2025 Dec;20(1):2491316. doi: 10.1080/15592294.2025.2491316. Epub 2025 Apr 16. Epigenetics. 2025. PMID: 40239010 Free PMC article. Review.
References
-
- American Cancer Society Key Statistics for Colorectal Cancer. [(accessed on 23 April 2024)]. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statis....
-
- International Agency for Research on Cancer. World Health Organization CANCER TODAY Data Visualization Tools for Exploring the Global Cancer Burden in 2020. [(accessed on 23 April 2024)]. Available online: https://gco.iarc.fr/today/home.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

