Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications
- PMID: 38892376
- PMCID: PMC11173118
- DOI: 10.3390/ijms25116187
Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications
Abstract
Cardiovascular disease represents the foremost cause of mortality and morbidity worldwide, with a steadily increasing incidence due to the growth of the ageing population. Cardiac dysfunction leading to heart failure may arise from acute myocardial infarction (MI) as well as inflammatory- and cancer-related chronic cardiomyopathy. Despite pharmacological progress, effective cardiac repair represents an unmet clinical need, with heart transplantation being the only option for end-stage heart failure. The functional profiling of the biological activity of extracellular vesicles (EVs) has recently attracted increasing interest in the field of translational research for cardiac regenerative medicine. The cardioprotective and cardioactive potential of human progenitor stem/cell-derived EVs has been reported in several preclinical studies, and EVs have been suggested as promising paracrine therapy candidates for future clinical translation. Nevertheless, some compelling aspects must be properly addressed, including optimizing delivery strategies to meet patient needs and enhancing targeting specificity to the cardiac tissue. Therefore, in this review, we will discuss the most relevant aspects of the therapeutic potential of EVs released by human progenitors for cardiovascular disease, with a specific focus on the strategies that have been recently implemented to improve myocardial targeting and administration routes.
Keywords: delivery; extracellular vesicle; heart disease; myocardial targeting; paracrine effect; sustained release.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
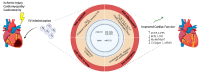

Similar articles
-
Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine.Cells. 2020 Mar 13;9(3):707. doi: 10.3390/cells9030707. Cells. 2020. PMID: 32183102 Free PMC article. Review.
-
Native and bioengineered extracellular vesicles for cardiovascular therapeutics.Nat Rev Cardiol. 2020 Nov;17(11):685-697. doi: 10.1038/s41569-020-0389-5. Epub 2020 Jun 1. Nat Rev Cardiol. 2020. PMID: 32483304 Free PMC article. Review.
-
Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases.Int J Mol Sci. 2021 Mar 19;22(6):3130. doi: 10.3390/ijms22063130. Int J Mol Sci. 2021. PMID: 33808520 Free PMC article. Review.
-
Extracellular Vesicles Released by Allogeneic Human Cardiac Stem/Progenitor Cells as Part of Their Therapeutic Benefit.Stem Cells Transl Med. 2019 Sep;8(9):911-924. doi: 10.1002/sctm.18-0256. Epub 2019 Mar 28. Stem Cells Transl Med. 2019. PMID: 30924311 Free PMC article.
-
Characterization of βARKct engineered cellular extracellular vesicles and model specific cardioprotection.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1276-H1289. doi: 10.1152/ajpheart.00571.2020. Epub 2021 Jan 29. Am J Physiol Heart Circ Physiol. 2021. PMID: 33513081 Free PMC article.
Cited by
-
Novel idebenone derivatives attenuated oxidative stress injury and myocardial damage.Front Chem. 2025 Feb 24;13:1544616. doi: 10.3389/fchem.2025.1544616. eCollection 2025. Front Chem. 2025. PMID: 40065841 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

