The Skin-Brain Axis: From UV and Pigmentation to Behaviour Modulation
- PMID: 38892387
- PMCID: PMC11172643
- DOI: 10.3390/ijms25116199
The Skin-Brain Axis: From UV and Pigmentation to Behaviour Modulation
Abstract
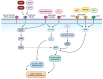
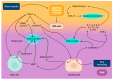
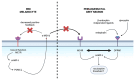
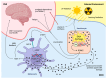
The skin-brain axis has been suggested to play a role in several pathophysiological conditions, including opioid addiction, Parkinson's disease and many others. Recent evidence suggests that pathways regulating skin pigmentation may directly and indirectly regulate behaviour. Conversely, CNS-driven neural and hormonal responses have been demonstrated to regulate pigmentation, e.g., under stress. Additionally, due to the shared neuroectodermal origins of the melanocytes and neurons in the CNS, certain CNS diseases may be linked to pigmentation-related changes due to common regulators, e.g., MC1R variations. Furthermore, the HPA analogue of the skin connects skin pigmentation to the endocrine system, thereby allowing the skin to index possible hormonal abnormalities visibly. In this review, insight is provided into skin pigment production and neuromelanin synthesis in the brain and recent findings are summarised on how signalling pathways in the skin, with a particular focus on pigmentation, are interconnected with the central nervous system. Thus, this review may supply a better understanding of the mechanism of several skin-brain associations in health and disease.
Keywords: ACTH; Addison’s disease; HPA axis; MC1R; MITF; MSH; POMC; Parkinson’s disease; UVR; central nervous system; cortisol; eumelanin; keratinocyte; melanocyte; melanogenesis; melanoma; neuromelanin; nociception; opioid signalling; pheomelanin; pigmentation; redhead; skin; skin–brain axis; tyrosinase; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lopez-Ojeda W., Pandey A., Alhajj M., Oakley A.M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Anatomy, Skin (Integument) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

