A Phyto-mycotherapeutic Supplement, Namely Ganostile, as Effective Adjuvant in Brain Cancer Management: An In Vitro Study Using U251 Human Glioblastoma Cell Line
- PMID: 38892392
- PMCID: PMC11172483
- DOI: 10.3390/ijms25116204
A Phyto-mycotherapeutic Supplement, Namely Ganostile, as Effective Adjuvant in Brain Cancer Management: An In Vitro Study Using U251 Human Glioblastoma Cell Line
Abstract
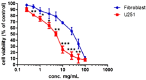
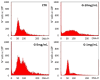
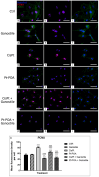
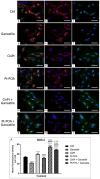
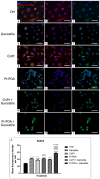
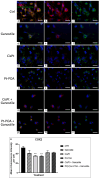
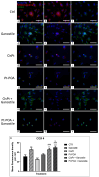
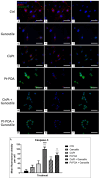
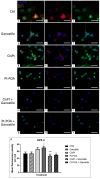
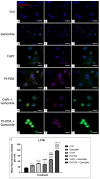
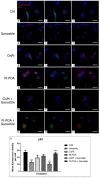
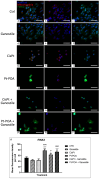
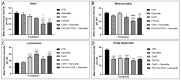
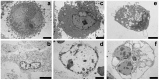
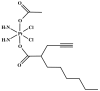
The current standard oncotherapy for glioblastoma is limited by several adverse side effects, leading to a short-term patient survival rate paralleled by a worsening quality of life (QoL). Recently, Complementary and Integrative Medicine's (CIM) innovative approaches have shown positive impacts in terms of better response to treatment, side effect reduction, and QoL improvement. In particular, promising potential in cancer therapy has been found in compounds coming from phyto- and mycotherapy. The objective of this study was to demonstrate the beneficial effects of a new phyto-mycotherapy supplement, named Ganostile, in the human glioblastoma cell line U251, in combination with chemotherapeutic agents, i.e., Cisplatin and a new platinum-based prodrug. Choosing a supplement dosage that mimicked oral supplementation in humans (about 1 g/day), through in vitro assays, microscopy, and cytometric analysis, it has emerged that the cells, after 48hr continuous exposure to Ganostile in combination with the chemical compounds, showed a higher mortality and a lower proliferation rate than the samples subjected to the different treatments administered individually. In conclusion, our data support the use of Ganostile in integrative oncology protocols as a promising adjuvant able to amplify conventional and new drug effects and also reducing resistance mechanisms often observed in brain tumors.
Keywords: drug resistance; ferroptosis; glioblastoma; mitochondrial disfunction; oxidative stress; phytotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Study on the activation of cell death mechanisms: in search of new therapeutic targets in glioblastoma multiforme.Apoptosis. 2023 Aug;28(7-8):1241-1257. doi: 10.1007/s10495-023-01857-x. Epub 2023 May 27. Apoptosis. 2023. PMID: 37244884 Free PMC article.
-
Silencing of Bmi-1 gene enhances chemotherapy sensitivity in human glioblastoma cells.Med Sci Monit. 2015 Apr 6;21:1002-7. doi: 10.12659/MSM.893754. Med Sci Monit. 2015. PMID: 25858624 Free PMC article.
-
Glioblastoma Therapy Using Codelivery of Cisplatin and Glutathione Peroxidase Targeting siRNA from Iron Oxide Nanoparticles.ACS Appl Mater Interfaces. 2020 Sep 30;12(39):43408-43421. doi: 10.1021/acsami.0c12042. Epub 2020 Sep 17. ACS Appl Mater Interfaces. 2020. PMID: 32885649
-
Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines.J Neurosurg. 2015 Mar;122(3):547-56. doi: 10.3171/2014.10.JNS14759. Epub 2014 Dec 19. J Neurosurg. 2015. PMID: 25526274
-
Development of a Prodrug of Camptothecin for Enhanced Treatment of Glioblastoma Multiforme.Mol Pharm. 2021 Apr 5;18(4):1558-1572. doi: 10.1021/acs.molpharmaceut.0c00968. Epub 2021 Mar 1. Mol Pharm. 2021. PMID: 33645231 Free PMC article.
Cited by
-
Towards Effective Treatment of Glioblastoma: The Role of Combination Therapies and the Potential of Phytotherapy and Micotherapy.Curr Issues Mol Biol. 2024 Dec 19;46(12):14324-14350. doi: 10.3390/cimb46120859. Curr Issues Mol Biol. 2024. PMID: 39727987 Free PMC article. Review.
-
Platinum(IV) anticancer therapies and cathepsin B: innovative strategies for overcoming resistance in glioblastoma cells.Front Cell Dev Biol. 2025 Jun 4;13:1506206. doi: 10.3389/fcell.2025.1506206. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40535571 Free PMC article.
-
Mechanistic insights into the therapeutic potential of β-elemene on glioma and other central nervous system diseases.Med Oncol. 2025 Aug 22;42(10):438. doi: 10.1007/s12032-025-03009-4. Med Oncol. 2025. PMID: 40846815 Review.
-
Examination of PDK1/AKT/mTOR transcription and exosomal mRNA levels in human glioblastoma cell line treated with a combination of temozolomide and hesperidin.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 15. doi: 10.1007/s00210-025-04137-4. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40372477
References
-
- Rossi P., Difrancia R., Quagliariello V., Savino E., Tralongo P., Randazzo C.L., Berretta M. B-Glucans from Grifola Frondosa and Ganoderma Lucidum in Breast Cancer: An Example of Complementary and Integrative Medicine. Oncotarget. 2018;9:24837–24856. doi: 10.18632/oncotarget.24984. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

