Arrhythmogenic Ventricular Remodeling by Next-Generation Bruton's Tyrosine Kinase Inhibitor Acalabrutinib
- PMID: 38892396
- PMCID: PMC11173147
- DOI: 10.3390/ijms25116207
Arrhythmogenic Ventricular Remodeling by Next-Generation Bruton's Tyrosine Kinase Inhibitor Acalabrutinib
Abstract
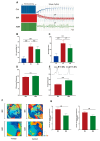
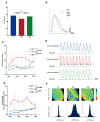
Cardiac arrhythmias remain a significant concern with Ibrutinib (IBR), a first-generation Bruton's tyrosine kinase inhibitor (BTKi). Acalabrutinib (ABR), a next-generation BTKi, is associated with reduced atrial arrhythmia events. However, the role of ABR in ventricular arrhythmia (VA) has not been adequately evaluated. Our study aimed to investigate VA vulnerability and ventricular electrophysiology following chronic ABR therapy in male Sprague-Dawley rats utilizing epicardial optical mapping for ventricular voltage and Ca2+ dynamics and VA induction by electrical stimulation in ex-vivo perfused hearts. Ventricular tissues were snap-frozen for protein analysis for sarcoplasmic Ca2+ and metabolic regulatory proteins. The results show that both ABR and IBR treatments increased VA vulnerability, with ABR showing higher VA regularity index (RI). IBR, but not ABR, is associated with the abbreviation of action potential duration (APD) and APD alternans. Both IBR and ABR increased diastolic Ca2+ leak and Ca2+ alternans, reduced conduction velocity (CV), and increased CV dispersion. Decreased SERCA2a expression and AMPK phosphorylation were observed with both treatments. Our results suggest that ABR treatment also increases the risk of VA by inducing proarrhythmic changes in Ca2+ signaling and membrane electrophysiology, as seen with IBR. However, the different impacts of these two BTKi on ventricular electrophysiology may contribute to differences in VA vulnerability and distinct VA characteristics.
Keywords: acalabrutinib; action potential; calcium cycling; electrical remodeling; ibrutinib; ventricular arrhythmia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Impaired Cardiac AMPK (5'-Adenosine Monophosphate-Activated Protein Kinase) and Ca2+-Handling, and Action Potential Duration Heterogeneity in Ibrutinib-Induced Ventricular Arrhythmia Vulnerability.J Am Heart Assoc. 2024 Jun 18;13(12):e032357. doi: 10.1161/JAHA.123.032357. Epub 2024 Jun 6. J Am Heart Assoc. 2024. PMID: 38842296 Free PMC article.
-
Ibrutinib, a Bruton's tyrosine kinase inhibitor, regulates ventricular electromechanical activities and enhances arrhythmogenesis.Eur J Pharmacol. 2024 Aug 15;977:176675. doi: 10.1016/j.ejphar.2024.176675. Epub 2024 May 31. Eur J Pharmacol. 2024. PMID: 38825303
-
Bruton's tyrosine kinase (BTK) inhibitors alter blood glucose and insulin in obese mice but reduce inflammation independent of BTK.Am J Physiol Endocrinol Metab. 2024 Sep 1;327(3):E271-E278. doi: 10.1152/ajpendo.00205.2024. Epub 2024 Jul 17. Am J Physiol Endocrinol Metab. 2024. PMID: 39017678
-
Treatment of Chronic Lymphocytic Leukemia After Discontinuation of Bruton's Tyrosine Kinase Inhibitors.Hematol Oncol Clin North Am. 2021 Aug;35(4):793-806. doi: 10.1016/j.hoc.2021.03.008. Epub 2021 May 27. Hematol Oncol Clin North Am. 2021. PMID: 34174986 Review.
-
Dermatological Toxicities of Bruton's Tyrosine Kinase Inhibitors.Am J Clin Dermatol. 2020 Dec;21(6):799-812. doi: 10.1007/s40257-020-00535-x. Am J Clin Dermatol. 2020. PMID: 32613545 Review.
Cited by
-
Epidemiology, clinical characteristics and potential mechanism of ibrutinib-induced ventricular arrhythmias.Front Pharmacol. 2024 Nov 19;15:1513913. doi: 10.3389/fphar.2024.1513913. eCollection 2024. Front Pharmacol. 2024. PMID: 39629084 Free PMC article. Review.
References
-
- Hillmen P., Pitchford A., Bloor A., Broom A., Young M., Kennedy B., Walewska R., Furtado M., Preston G., Neilson J.R., et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): Interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24:535–552. doi: 10.1016/S1470-2045(23)00144-4. - DOI - PubMed
-
- Quartermaine C., Ghazi S.M., Yasin A., Awan F.T., Fradley M., Wiczer T., Kalathoor S., Ferdousi M., Krishan S., Habib A., et al. Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. JACC Cardio Oncol. 2023;5:570–590. doi: 10.1016/j.jaccao.2023.09.002. - DOI - PMC - PubMed
-
- Mato A.R., Nabhan C., Thompson M.C., Lamanna N., Brander D.M., Hill B., Howlett C., Skarbnik A., Cheson B.D., Zent C., et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica. 2018;103:874–879. doi: 10.3324/haematol.2017.182907. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

