Does Cell-Type-Specific Silencing of Monoamine Oxidase B Interfere with the Development of Right Ventricle (RV) Hypertrophy or Right Ventricle Failure in Pulmonary Hypertension?
- PMID: 38892401
- PMCID: PMC11172614
- DOI: 10.3390/ijms25116212
Does Cell-Type-Specific Silencing of Monoamine Oxidase B Interfere with the Development of Right Ventricle (RV) Hypertrophy or Right Ventricle Failure in Pulmonary Hypertension?
Abstract
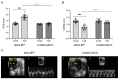
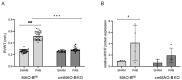
Increased mitochondrial reactive oxygen species (ROS) formation is important for the development of right ventricular (RV) hypertrophy (RVH) and failure (RVF) during pulmonary hypertension (PH). ROS molecules are produced in different compartments within the cell, with mitochondria known to produce the strongest ROS signal. Among ROS-forming mitochondrial proteins, outer-mitochondrial-membrane-located monoamine oxidases (MAOs, type A or B) are capable of degrading neurotransmitters, thereby producing large amounts of ROS. In mice, MAO-B is the dominant isoform, which is present in almost all cell types within the heart. We analyzed the effect of an inducible cardiomyocyte-specific knockout of MAO-B (cmMAO-B KO) for the development of RVH and RVF in mice. Right ventricular hypertrophy was induced by pulmonary artery banding (PAB). RV dimensions and function were measured through echocardiography. ROS production (dihydroethidium staining), protein kinase activity (PamStation device), and systemic hemodynamics (in vivo catheterization) were assessed. A significant decrease in ROS formation was measured in cmMAO-B KO mice during PAB compared to Cre-negative littermates, which was associated with reduced activity of protein kinases involved in hypertrophic growth. In contrast to littermates in which the RV was dilated and hypertrophied following PAB, RV dimensions were unaffected in response to PAB in cmMAO-B KO mice, and no decline in RV systolic function otherwise seen in littermates during PAB was measured in cmMAO-B KO mice. In conclusion, cmMAO-B KO mice are protected against RV dilatation, hypertrophy, and dysfunction following RV pressure overload compared to littermates. These results support the hypothesis that cmMAO-B is a key player in causing RV hypertrophy and failure during PH.
Keywords: monoamine oxidase; pulmonary hypertension; right heart.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Heusch P., Canton M., Aker S., Van De Sand A., Konietzka I., Rassaf T., Menazza S., Brodde O., Di Lisa F., Heusch G., et al. The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br. J. Pharmacol. 2010;160:1408–1416. doi: 10.1111/j.1476-5381.2010.00793.x. - DOI - PMC - PubMed
-
- Andreadou I., Schulz R., Papapetropoulos A., Turan B., Ytrehus K., Ferdinandy P., Daiber A., Di Lisa F. The role of mitochondrial reactive oxygen species, NO and H 2 S in ischaemia/reperfusion injury and cardioprotection. J. Cell. Mol. Med. 2020;24:6510–6522. doi: 10.1111/jcmm.15279. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

