Extrusion of Neutrophil Extracellular Traps (NETs) Negatively Impacts Canine Sperm Functions: Implications in Reproductive Failure
- PMID: 38892404
- PMCID: PMC11172674
- DOI: 10.3390/ijms25116216
Extrusion of Neutrophil Extracellular Traps (NETs) Negatively Impacts Canine Sperm Functions: Implications in Reproductive Failure
Abstract
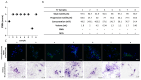
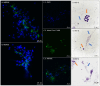
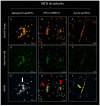
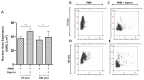
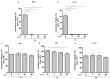
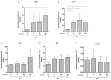
Reproductive failure in dogs is often due to unknown causes, and correct diagnosis and treatment are not always achieved. This condition is associated with various congenital and acquired etiologies that develop inflammatory processes, causing an increase in the number of leukocytes within the female reproductive tract (FRT). An encounter between polymorphonuclear neutrophils (PMNs) and infectious agents or inflammation in the FRT could trigger neutrophil extracellular traps (NETs), which are associated with significantly decreased motility and damage to sperm functional parameters in other species, including humans. This study describes the interaction between canine PMNs and spermatozoa and characterizes the release of NETs, in addition to evaluating the consequences of these structures on canine sperm function. To identify and visualize NETs, May-Grünwald Giemsa staining and immunofluorescence for neutrophil elastase (NE) were performed on canine semen samples and sperm/PMN co-cultures. Sperm viability was assessed using SYBR/PI and acrosome integrity was assessed using PNA-FITC/PI by flow cytometry. The results demonstrate NETs release in native semen samples and PMN/sperm co-cultures. In addition, NETs negatively affect canine sperm function parameters. This is the first report on the ability of NETs to efficiently entrap canine spermatozoa, and to provide additional data on the adverse effects of NETs on male gametes. Therefore, NETs formation should be considered in future studies of canine reproductive failure, as these extracellular fibers and NET-derived pro-inflammatory capacities will impede proper oocyte fertilization and embryo implantation. These data will serve as a basis to explain certain reproductive failures of dogs and provide new information about triggers and molecules involved in adverse effects of NETosis for domestic pet animals.
Keywords: PMNs; canine; neutrophil extracellular traps (NETs); sperm functionality; spermatozoon.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility.Fertil Steril. 2016 Oct;106(5):1053-1060.e1. doi: 10.1016/j.fertnstert.2016.06.005. Epub 2016 Jun 23. Fertil Steril. 2016. PMID: 27344301
-
Effect of different sperm populations on neutrophils extracellular traps (NETs) formation in cattle.Res Vet Sci. 2023 Nov;164:105028. doi: 10.1016/j.rvsc.2023.105028. Epub 2023 Sep 22. Res Vet Sci. 2023. PMID: 37804665
-
Bovine sperm samples induce different NET phenotypes in a NADPH oxidase-, PAD4-, and Ca++-dependent process†.Biol Reprod. 2020 Apr 15;102(4):902-914. doi: 10.1093/biolre/ioaa003. Biol Reprod. 2020. PMID: 31967293
-
Interaction of sperm cells with the female reproductive tract in cattle: Focus on neutrophil extracellular trap formation.Anim Reprod Sci. 2022 Nov;246:107056. doi: 10.1016/j.anireprosci.2022.107056. Epub 2022 Aug 20. Anim Reprod Sci. 2022. PMID: 36031509 Review.
-
Neutrophil Elastase and Neutrophil Extracellular Traps in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1263:13-23. doi: 10.1007/978-3-030-44518-8_2. Adv Exp Med Biol. 2020. PMID: 32588320 Free PMC article. Review.
Cited by
-
Neutrophil Extracellular Traps (NETs) in Immunity and Diseases: Second Edition.Int J Mol Sci. 2025 Jul 8;26(14):6563. doi: 10.3390/ijms26146563. Int J Mol Sci. 2025. PMID: 40724813 Free PMC article.
-
Impact of hyperglycaemia on cellular microenvironment and function of endometrium and uterine tube: scoping review focused on infertility in diabetic women.Front Cell Dev Biol. 2025 May 23;13:1582039. doi: 10.3389/fcell.2025.1582039. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40486907 Free PMC article. Review.
-
The FKBPL-based therapeutic peptide, AD-01, protects the endothelium from hypoxia-induced damage by stabilising hypoxia inducible factor-α and inflammation.J Transl Med. 2025 Mar 11;23(1):309. doi: 10.1186/s12967-025-06118-w. J Transl Med. 2025. PMID: 40069829 Free PMC article.
References
-
- Fontbonne A. Infertility in Male Dogs. Mod. Vet. Pract. 2011;58:1038–1039. - PubMed
-
- Johnston S.D., Kustritz M.V., Olson P.S. Canine and Feline Theriogenology. Volume I Saunders; Philadelphia, PA, USA: London, UK: 2001.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

