Long-Term High-Fat Diet Limits the Protective Effect of Spontaneous Physical Activity on Mammary Carcinogenesis
- PMID: 38892407
- PMCID: PMC11172547
- DOI: 10.3390/ijms25116221
Long-Term High-Fat Diet Limits the Protective Effect of Spontaneous Physical Activity on Mammary Carcinogenesis
Abstract
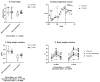
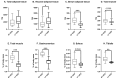
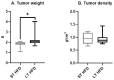
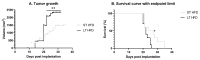
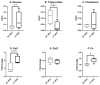
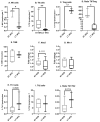
Breast cancer is influenced by factors such as diet, a sedentary lifestyle, obesity, and postmenopausal status, which are all linked to prolonged hormonal and inflammatory exposure. Physical activity offers protection against breast cancer by modulating hormones, immune responses, and oxidative defenses. This study aimed to assess how a prolonged high-fat diet (HFD) affects the effectiveness of physical activity in preventing and managing mammary tumorigenesis. Ovariectomised C57BL/6 mice were provided with an enriched environment to induce spontaneous physical activity while being fed HFD. After 44 days (short-term, ST HFD) or 88 days (long-term, LT HFD), syngenic EO771 cells were implanted into mammary glands, and tumour growth was monitored until sacrifice. Despite similar physical activity and food intake, the LT HFD group exhibited higher visceral adipose tissue mass and reduced skeletal muscle mass. In the tumour microenvironment, the LT HFD group showed decreased NK cells and TCD8+ cells, with a trend toward increased T regulatory cells, leading to a collapse of the T8/Treg ratio. Additionally, the LT HFD group displayed decreased tumour triglyceride content and altered enzyme activities indicative of oxidative stress. Prolonged exposure to HFD was associated with tumour growth despite elevated physical activity, promoting a tolerogenic tumour microenvironment. Future studies should explore inter-organ exchanges between tumour and tissues.
Keywords: high-fat diet; immunity; mammary carcinogenesis; obesity; oxidative stress; spontaneous physical activity; tumour microenvironment.
Conflict of interest statement
None of the authors have any conflicts of interest to report. The authors are responsible for the content and writing of the paper.
Figures

References
-
- Ferlay J., Ervik M., Laversanne M., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. [(accessed on 21 February 2024)]. Available online: https://gco.iarc.who.int/today.
-
- Tung N., Lin N.U., Kidd J., Allen B.A., Singh N., Wenstrup R.J., Hartman A.-R., Winer E.P., Garber J.E. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J. Clin. Oncol. 2024;34:1460. doi: 10.1200/JCO.2015.65.0747. - DOI - PMC - PubMed
-
- Möller S., Mucci L.A., Harris J.R., Scheike T., Holst K., Halekoh U., Adami H.-O., Czene K., Christensen K., Holm N.V., et al. The Heritability of Breast Cancer among Women in the Nordic Twin Study of Cancer. Cancer Epidemiol. Biomark. Prev. 2016;25:145–150. doi: 10.1158/1055-9965.EPI-15-0913. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

