Triple Blockade of Oncogenic RAS Signaling Using KRAS and MEK Inhibitors in Combination with Irradiation in Pancreatic Cancer
- PMID: 38892436
- PMCID: PMC11172716
- DOI: 10.3390/ijms25116249
Triple Blockade of Oncogenic RAS Signaling Using KRAS and MEK Inhibitors in Combination with Irradiation in Pancreatic Cancer
Abstract
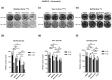
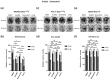
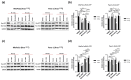
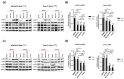
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest of human malignancies and carries an exceptionally poor prognosis. It is mostly driven by multiple oncogenic alterations, with the highest mutation frequency being observed in the KRAS gene, which is a key oncogenic driver of tumorogenesis and malignant progression in PDAC. However, KRAS remained undruggable for decades until the emergence of G12C mutation specific KRAS inhibitors. Despite this development, this therapeutic approach to target KRAS directly is not routinely used for PDAC patients, with the reasons being the rare presence of G12C mutation in PDAC with only 1-2% of occurring cases, modest therapeutic efficacy, activation of compensatory pathways leading to cell resistance, and absence of effective KRASG12D or pan-KRAS inhibitors. Additionally, indirect approaches to targeting KRAS through upstream and downstream regulators or effectors were also found to be either ineffective or known to cause major toxicities. For this reason, new and more effective treatment strategies that combine different therapeutic modalities aiming at achieving synergism and minimizing intrinsic or adaptive resistance mechanisms are required. In the current work presented here, pancreatic cancer cell lines with oncogenic KRAS G12C, G12D, or wild-type KRAS were treated with specific KRAS or SOS1/2 inhibitors, and therapeutic synergisms with concomitant MEK inhibition and irradiation were systematically evaluated by means of cell viability, 2D-clonogenic, 3D-anchorage independent soft agar, and bioluminescent ATP assays. Underlying pathophysiological mechanisms were examined by using Western blot analyses, apoptosis assay, and RAS activation assay.
Keywords: KRAS inhibitors; MEK inhibitor; RAS/MAPK pathway; combination therapy; irradiation; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Concurrent inhibition of pBADS99 synergistically improves MEK inhibitor efficacy in KRASG12D-mutant pancreatic ductal adenocarcinoma.Cell Death Dis. 2024 Feb 26;15(2):173. doi: 10.1038/s41419-024-06551-7. Cell Death Dis. 2024. PMID: 38409090 Free PMC article.
-
ADT-1004: a first-in-class, oral pan-RAS inhibitor with robust antitumor activity in preclinical models of pancreatic ductal adenocarcinoma.Mol Cancer. 2025 Mar 13;24(1):76. doi: 10.1186/s12943-025-02288-9. Mol Cancer. 2025. PMID: 40082968 Free PMC article.
-
Jorunnamycin A induces apoptosis in pancreatic ductal adenocarcinoma cells, spheroids, and patient-derived organoids by modulating KRAS-mediated survival pathways.Sci Rep. 2025 Apr 3;15(1):11376. doi: 10.1038/s41598-025-95766-5. Sci Rep. 2025. PMID: 40175514 Free PMC article.
-
Drugging the 'undruggable' KRAS: breakthroughs, challenges, and opportunities in pancreatic cancer.Cancer Biol Med. 2025 Jul 7;22(7):762-88. doi: 10.20892/j.issn.2095-3941.2025.0122. Cancer Biol Med. 2025. PMID: 40624835 Free PMC article. Review.
-
KRAS-Dependency in Pancreatic Ductal Adenocarcinoma: Mechanisms of Escaping in Resistance to KRAS Inhibitors and Perspectives of Therapy.Int J Mol Sci. 2023 May 26;24(11):9313. doi: 10.3390/ijms24119313. Int J Mol Sci. 2023. PMID: 37298264 Free PMC article. Review.
Cited by
-
Synergistic anticancer effects of camptothecin and sotorasib in KRAS-mutated pancreatic ductal adenocarcinoma.Front Pharmacol. 2025 Jul 18;16:1635449. doi: 10.3389/fphar.2025.1635449. eCollection 2025. Front Pharmacol. 2025. PMID: 40756996 Free PMC article.
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Bsc M.F.B., Me J.F., Soerjomataram M.I., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

