Comprehensive Insights into the Remarkable Function and Regulatory Mechanism of FluG during Asexual Development in Beauveria bassiana
- PMID: 38892450
- PMCID: PMC11173134
- DOI: 10.3390/ijms25116261
Comprehensive Insights into the Remarkable Function and Regulatory Mechanism of FluG during Asexual Development in Beauveria bassiana
Abstract
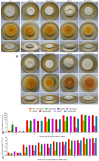
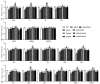
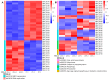
Asexual development is the main propagation and transmission mode of Beauveria bassiana and the basis of its pathogenicity. The regulation mechanism of conidiation and the key gene resources for utilization are key links to improving the conidia yield and quality of Beauveria bassiana. Their clarification may promote the industrialization of fungal pesticides. Here, we compared the regulation of morphology, resistance to external stress, virulence, and nutrient utilization capacity between the upstream developmental regulatory gene fluG and the key genes brlA, abaA, and wetA in the central growth and development pathway. The results showed that the ΔbrlA and ΔabaA mutants completely lost the capacity to conidiate and that the ΔwetA mutant had seriously reduced conidiation capacity. Although the deletion of fluG did not reduce the conidiation ability as much as deletions of brlA, abaA, and wetA, it significantly reduced the fungal response to external stress, virulence, and nutrient utilization, while the deletion of the three other genes had little effect. Via transcriptome analysis and screening the yeast nuclear system library, we found that the differentially expressed genes in the ΔfluG mutants were concentrated in the signaling pathways of ABC transporters, propionate metabolism, tryptophan metabolism, DNA replication, mismatch repair, and fatty acid metabolism. FluG directly acted on 40 proteins that were involved in various signaling pathways such as metabolism, oxidative stress, and cell homeostasis. The analysis indicated that the regulatory function of fluG was mainly involved in DNA replication, cell homeostasis, fungal growth and metabolism, and the response to external stress. Our results revealed the biological function of fluG in asexual development and the responses to several environmental stresses as well as its influence on the asexual development regulatory network in B. bassiana.
Keywords: Beauveria bassiana; asexual development; protein interaction; signaling pathway; upstream developmental regulatory protein; virulence.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
C-terminal Ser/Thr residues are vital for the regulatory role of Ste7 in the asexual cycle and virulence of Beauveria bassiana.Appl Microbiol Biotechnol. 2018 Aug;102(16):6973-6986. doi: 10.1007/s00253-018-9148-5. Epub 2018 Jun 11. Appl Microbiol Biotechnol. 2018. PMID: 29948113
-
WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen.Appl Microbiol Biotechnol. 2015 Dec;99(23):10069-81. doi: 10.1007/s00253-015-6823-7. Epub 2015 Aug 5. Appl Microbiol Biotechnol. 2015. PMID: 26243054
-
The velvet protein VeA functions in asexual cycle, stress tolerance and transcriptional regulation of Beauveria bassiana.Fungal Genet Biol. 2019 Jun;127:1-11. doi: 10.1016/j.fgb.2019.02.009. Epub 2019 Feb 23. Fungal Genet Biol. 2019. PMID: 30807832
-
Molecular Genetics of Beauveria bassiana Infection of Insects.Adv Genet. 2016;94:165-249. doi: 10.1016/bs.adgen.2015.11.003. Epub 2016 Feb 11. Adv Genet. 2016. PMID: 27131326 Review.
-
Regulatory functions of homeobox domain transcription factors in fungi.Appl Environ Microbiol. 2024 Mar 20;90(3):e0220823. doi: 10.1128/aem.02208-23. Epub 2024 Feb 29. Appl Environ Microbiol. 2024. PMID: 38421174 Free PMC article. Review.
References
-
- Wang C.S., Feng M.G. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol. Control. 2014;68:129–135. doi: 10.1016/j.biocontrol.2013.06.017. - DOI
-
- Pedrini N., Ortiz-Urquiza A., Huarte-Bonnet C., Zhang S., Keyhani N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013;4:24. doi: 10.3389/fmicb.2013.00024. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

