Diagnostic Utility of Selected Matrix Metalloproteinases (MMP-2, MMP-3, MMP-11, MMP-26), HE4, CA125 and ROMA Algorithm in Diagnosis of Ovarian Cancer
- PMID: 38892452
- PMCID: PMC11173327
- DOI: 10.3390/ijms25116265
Diagnostic Utility of Selected Matrix Metalloproteinases (MMP-2, MMP-3, MMP-11, MMP-26), HE4, CA125 and ROMA Algorithm in Diagnosis of Ovarian Cancer
Abstract
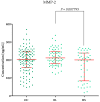
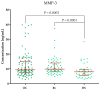
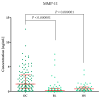
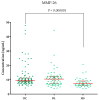
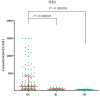
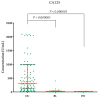
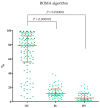
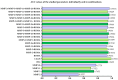
Ovarian cancer (OC) has an unfavorable prognosis. Due to the lack of effective screening tests, new diagnostic methods are being sought to detect OC earlier. The aim of this study was to evaluate the concentration and diagnostic utility of selected matrix metalloproteinases (MMPs) as OC markers in comparison with HE4, CA125 and the ROMA algorithm. The study group consisted of 120 patients with OC; the comparison group consisted of 70 patients with benign lesions and 50 healthy women. MMPs were determined via the ELISA method, HE4 and CA125 by CMIA. Patients with OC had elevated levels of MMP-3 and MMP-11, similar to HE4, CA125 and ROMA values. The highest SE, SP, NPV and PPV values were found for MMP-26, CA125 and ROMA in OC patients. Performing combined analyses of ROMA with selected MMPs increased the values of diagnostic parameters. The topmost diagnostic power of the test was obtained for MMP-26, CA125, HE4 and ROMA and performing combined analyses of MMPs and ROMA enhanced the diagnostic power of the test. The obtained results indicate that the tested MMPs do not show potential as stand-alone OC biomarkers, but can be considered as additional tests to raise the diagnostic utility of the ROMA algorithm.
Keywords: CA125; HE4; MMP-11; MMP-2; MMP-26; MMP-3; ROMA algorithm; Serous cystadenomas; ovarian cancer; plasma concentration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Ovarian Cancer Coalition World Ovarian Cancer Coalition Atlas 2023. [(accessed on 17 April 2024)]. Available online: https://worldovariancancercoalition.org/wp-content/uploads/2023/03/World....
-
- Ravindran F., Choudhary B. Ovarian Cancer-Updates in Tumour Biology and Therapeutics. IntechOpen; Rijeka, Croatia: 2021. Ovarian cancer: Molecular classification and targeted therapy; pp. 1–21.
-
- Dilley J., Gentry-Maharaj A., Ryan A., Burnell M., Manchanda R., Kalsi J., Singh N., Woolas R., Sharma A., Williamson K., et al. Ovarian Cancer Symptoms in Pre-Clinical Invasive Epithelial Ovarian Cancer—An Exploratory Analysis Nested within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Gynecol. Oncol. 2023;179:123–130. doi: 10.1016/j.ygyno.2023.11.005. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

