The Expansion of Sirtuin Gene Family in Gilthead Sea Bream (Sparus aurata)-Phylogenetic, Syntenic, and Functional Insights across the Vertebrate/Fish Lineage
- PMID: 38892461
- PMCID: PMC11172991
- DOI: 10.3390/ijms25116273
The Expansion of Sirtuin Gene Family in Gilthead Sea Bream (Sparus aurata)-Phylogenetic, Syntenic, and Functional Insights across the Vertebrate/Fish Lineage
Abstract
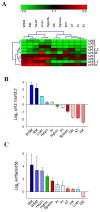
The Sirtuin (SIRT1-7) family comprises seven evolutionary-conserved enzymes that couple cellular NAD availability with health, nutrition and welfare status in vertebrates. This study re-annotated the sirt3/5 branch in the gilthead sea bream, revealing three paralogues of sirt3 (sirt3.1a/sirt3.1b/sirt3.2) and two of sirt5 (sirt5a/sirt5b) in this Perciform fish. The phylogeny and synteny analyses unveiled that the Sirt3.1/Sirt3.2 dichotomy was retained in teleosts and aquatic-living Sarcopterygian after early vertebrate 2R whole genome duplication (WGD). Additionally, only certain percomorphaceae and gilthead sea bream showed a conserved tandem-duplicated synteny block involving the mammalian-clustered sirt3.1 gene (psmd13-sirt3.1a/b-drd4-cdhr5-ctsd). Conversely, the expansion of the Sirt5 branch was shaped by the teleost-specific 3R WGD. As extensively reviewed in the literature, human-orthologues (sirt3.1/sirt5a) showed a high, conserved expression in skeletal muscle that increased as development advanced. However, recent sirt3.2 and sirt5b suffered an overall muscle transcriptional silencing across life, as well as an enhanced expression on immune-relevant tissues and gills. These findings fill gaps in the ontogeny and differentiation of Sirt genes in the environmentally adaptable gilthead sea bream, becoming a good starting point to advance towards a full understanding of its neo-functionalization. The mechanisms originating from these new paralogs also open new perspectives in the study of cellular energy sensing processes in vertebrates.
Keywords: adaptive plasticity; gene duplication; gilthead sea bream; neo-functionalization; phylogeny; sirtuin; synteny.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Tissue-specific gene expression and fasting regulation of sirtuin family in gilthead sea bream (Sparus aurata).J Comp Physiol B. 2017 Jan;187(1):153-163. doi: 10.1007/s00360-016-1014-0. Epub 2016 Jul 18. J Comp Physiol B. 2017. PMID: 27431591
-
Molecular characterization and expression analysis of six peroxiredoxin paralogous genes in gilthead sea bream (Sparus aurata): insights from fish exposed to dietary, pathogen and confinement stressors.Fish Shellfish Immunol. 2011 Aug;31(2):294-302. doi: 10.1016/j.fsi.2011.05.015. Epub 2011 May 27. Fish Shellfish Immunol. 2011. PMID: 21640832
-
Characterisation and expression analysis of cathepsins and ubiquitin-proteasome genes in gilthead sea bream (Sparus aurata) skeletal muscle.BMC Res Notes. 2015 Apr 15;8:149. doi: 10.1186/s13104-015-1121-0. BMC Res Notes. 2015. PMID: 25880457 Free PMC article.
-
A gene-based radiation hybrid map of the gilthead sea bream Sparus aurata refines and exploits conserved synteny with Tetraodon nigroviridis.BMC Genomics. 2007 Feb 7;8:44. doi: 10.1186/1471-2164-8-44. BMC Genomics. 2007. PMID: 17286862 Free PMC article.
-
New Insights Into the Evolutionary History of Melatonin Receptors in Vertebrates, With Particular Focus on Teleosts.Front Endocrinol (Lausanne). 2020 Sep 24;11:538196. doi: 10.3389/fendo.2020.538196. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33071966 Free PMC article. Review.
Cited by
-
Potential roles of the sirtuins in promoting longevity for larger Argopecten scallops.Mar Life Sci Technol. 2025 Mar 4;7(2):284-301. doi: 10.1007/s42995-024-00269-3. eCollection 2025 May. Mar Life Sci Technol. 2025. PMID: 40417254 Free PMC article.
-
Phylogenetic analysis and detection of positive selection in the SIRT gene family across vertebrates.Sci Rep. 2025 Jan 5;15(1):848. doi: 10.1038/s41598-025-85344-0. Sci Rep. 2025. PMID: 39757258 Free PMC article.
-
A century of anthropogenic perturbations impact genomic signatures of the iconic migratory Atlantic cod.Sci Adv. 2025 Aug;11(31):eadp3342. doi: 10.1126/sciadv.adp3342. Epub 2025 Jul 30. Sci Adv. 2025. PMID: 40737403 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

