Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis
- PMID: 38892586
- PMCID: PMC11174044
- DOI: 10.3390/nu16111653
Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis
Abstract
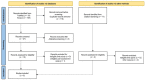
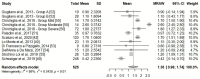
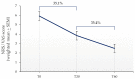
Palmitoylethanolamide (PEA) emerged over the years as a promising approach in the management of chronic pain. Despite the fact that the efficacy of micron-size PEA formulations appears to be time-dependent, the optimal timing has not yet been elucidated. This systematic review and meta-analysis aim to estimate the possible advantage of an extended treatment in the relief of chronic pain. The literature search was conducted consulting scientific databases, to identify clinical trials in which micron-size PEA was administered for at least 60 days, and pain assessed by the Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS). Nine studies matched the required criteria, for a total of 742 patients involved. The meta-analysis showed a statistically and clinically significant pain intensity reduction after 60 days of micron-size PEA supplementation, compared to 30 days (1.36 points, p < 0.01). The secondary analysis revealed a weighted NRS/VAS score decrease of 2.08 points within the first month of treatment. These two obtained scores corresponded to a 35.1% pain intensity reduction within the first month, followed by a further 35.4% during the second month. Overall, these results confirm the clinically relevant and time-depended pain-relieving effect of micron-size PEA and therefore the advantage of an extended treatment, especially in patient with incomplete pain management.
Keywords: (ultra-)micronization; chronic pain; chronic pelvic pain; micron-size PEA; neuroinflammation; palmitoylethanolamide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Latina R., De Marinis M.G., Giordano F., Osborn J.F., Giannarelli D., Di Biagio E., Varrassi G., Sansoni J., Bertini L., Baglio G., et al. Epidemiology of Chronic Pain in the Latium Region, Italy: A Cross-Sectional Study on the Clinical Characteristics of Patients Attending Pain Clinics. Pain. Manag. Nurs. 2019;20:373–381. doi: 10.1016/j.pmn.2019.01.005. - DOI - PubMed
-
- Artukoglu B.B., Beyer C., Zuloff-Shani A., Brener E., Howard Bloch M. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain. Physician. 2017;20:353–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

