Understanding the Impact of Different Doses of Reducose® Mulberry Leaf Extract on Blood Glucose and Insulin Responses after Eating a Complex Meal: Results from a Double-Blind, Randomised, Crossover Trial
- PMID: 38892603
- PMCID: PMC11174565
- DOI: 10.3390/nu16111670
Understanding the Impact of Different Doses of Reducose® Mulberry Leaf Extract on Blood Glucose and Insulin Responses after Eating a Complex Meal: Results from a Double-Blind, Randomised, Crossover Trial
Abstract
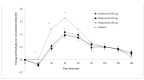
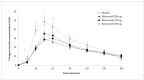
Non-communicable diseases (NCDs) are becoming an increasingly important health concern due to a rapidly ageing global population. The fastest growing NCD, type 2 diabetes mellitus (T2DM), is responsible for over 2 million deaths annually. Lifestyle changes, including dietary changes to low glycemic response (GR) foods, have been shown to reduce the risk of developing T2DM. The aim of this study was to investigate whether three different doses of Reducose®, a mulberry leaf extract, could lower the GR and insulinemic responses (IR) to a full meal challenge in healthy individuals. A double-blind, randomised, placebo-controlled, repeat-measure, crossover design trial was conducted by the Oxford Brookes Centre for Nutrition and Health; 37 healthy individuals completed the study. Participants consumed capsules containing either 200 mg, 225 mg, 250 mg Reducose® or placebo before a test meal consisting of 150 g white bread and egg mayo filler. Capillary blood samples were collected at 15-min intervals in the first hour and at 30-min intervals over the second and third hours to determine glucose and plasma insulin levels. The consumption of all three doses of Reducose® resulted in significantly lower blood glucose and plasma insulin levels compared to placebo. All three doses of Reducose® (200 mg, 225 mg, 250 mg) significantly lowered glucose iAUC 120 by 30% (p = 0.003), 33% (p = 0.001) and 32% (p = 0.002), respectively, compared with placebo. All three doses of Reducose® (200 mg, 225 mg, 250 mg) significantly lowered the plasma insulin iAUC 120 by 31% (p = 0.024), 34% (p = 0.004) and 38% (p < 0.001), respectively. The study demonstrates that the recommended dose (250 mg) and two lower doses (200 mg, 225 mg) of Reducose® can be used to help lower the GR and IR of a full meal containing carbohydrates, fats and proteins.
Keywords: 1-deoxynojirimycin; glycaemic response; insulinemic response; mulberry leaf extract.
Conflict of interest statement
A.G. and M.L. are employees of Phynova Group Limited. Phynova Group Limited was involved in the conception and design of the study but had no role in the collection or analysis of the data. A.G. and M.L. drafted the original draft manuscript.
Figures
References
-
- WHO . Noncommunicable Diseases. WHO; Geneva, Switzerland: 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

