Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines
- PMID: 38892725
- PMCID: PMC11174739
- DOI: 10.3390/nu16111792
Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines
Abstract
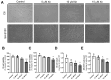
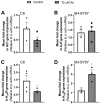
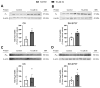
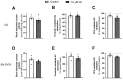
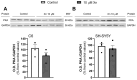
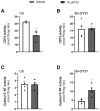
Xanthohumol (Xn) is an antioxidant flavonoid mainly extracted from hops (Humulus lupulus), one of the main ingredients of beer. As with other bioactive compounds, their therapeutic potential against different diseases has been tested, one of which is Alzheimer's disease (AD). Adenosine is a neuromodulatory nucleoside that acts through four different G protein-coupled receptors: A1 and A3, which inhibit the adenylyl cyclases (AC) pathway, and A2A and A2B, which stimulate this activity, causing either a decrease or an increase, respectively, in the release of excitatory neurotransmitters such as glutamate. This adenosinergic pathway, which is altered in AD, could be involved in the excitotoxicity process. Therefore, the aim of this work is to describe the effect of Xn on the adenosinergic pathway using cell lines. For this purpose, two different cellular models, rat glioma C6 and human neuroblastoma SH-SY5Y, were exposed to a non-cytotoxic 10 µM Xn concentration. Adenosine A1 and A2A, receptor levels, and activities related to the adenosine pathway, such as adenylate cyclase, protein kinase A, and 5'-nucleotidase, were analyzed. The adenosine A1 receptor was significantly increased after Xn exposure, while no changes in A2A receptor membrane levels or AC activity were reported. Regarding 5'-nucleotidases, modulation of their activity by Xn was noted since CD73, the extracellular membrane attached to 5'-nucleotidase, was significantly decreased in the C6 cell line. In conclusion, here we describe a novel pathway in which the bioactive flavonoid Xn could have potentially beneficial effects on AD as it increases membrane A1 receptors while modulating enzymes related to the adenosine pathway in cell cultures.
Keywords: adenosine receptors; cell cultures; xanthohumol.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Modulation of Adenosine Receptors by Hops and Xanthohumol in Cell Cultures.ACS Chem Neurosci. 2021 Jul 7;12(13):2373-2384. doi: 10.1021/acschemneuro.1c00130. Epub 2021 Jun 22. ACS Chem Neurosci. 2021. PMID: 34156813
-
Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus).Molecules. 2015 Jan 7;20(1):754-79. doi: 10.3390/molecules20010754. Molecules. 2015. PMID: 25574819 Free PMC article. Review.
-
The miR-204-3p-targeted IGFBP2 pathway is involved in xanthohumol-induced glioma cell apoptotic death.Neuropharmacology. 2016 Nov;110(Pt A):362-375. doi: 10.1016/j.neuropharm.2016.07.038. Epub 2016 Jul 31. Neuropharmacology. 2016. PMID: 27487563
-
Xanthohumol-Induced Rat Glioma C6 Cells Death by Triggering Mitochondrial Stress.Int J Mol Sci. 2021 Apr 26;22(9):4506. doi: 10.3390/ijms22094506. Int J Mol Sci. 2021. PMID: 33925918 Free PMC article.
-
Mechanism of Action and Therapeutic Potential of Xanthohumol in Prevention of Selected Neurodegenerative Diseases.Molecules. 2025 Feb 5;30(3):694. doi: 10.3390/molecules30030694. Molecules. 2025. PMID: 39942798 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- Grant PID2022-140602NB-100/Ministerio de Ciencia, Innovación y Universidades de España and FEDER
- grant 2022-GRIN-34201/University of Castilla-la Mancha cofinanced with FEDER
- grant SBPLY/23/180225/000156/Junta de Comunidades de Castilla-La Mancha
- CR-2023-103/A.T is a recipient of a predoctoral Manuel Oya 2022 fellowship from Fundación Cerveza y Salud (CR-2023-103).
LinkOut - more resources
Full Text Sources
Medical
Research Materials

