Physical Activity, Bleedings and Quality of Life in Subjects with Haemophilia A without Inhibitors-A Multicenter, Observational Italian Study with a Wearable Device
- PMID: 38892747
- PMCID: PMC11172795
- DOI: 10.3390/jcm13113036
Physical Activity, Bleedings and Quality of Life in Subjects with Haemophilia A without Inhibitors-A Multicenter, Observational Italian Study with a Wearable Device
Abstract
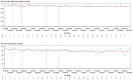
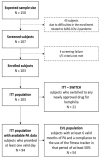
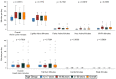
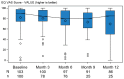
Background: This study aimed to gather data on physical activity (PA), bleeding, health-related quality of life, and health status, using a wearable device and an electronic patient-reported outcome (ePRO) app, in individuals with moderate or severe hemophilia A (HA) without inhibitors receiving treatment according to the clinical practice. Methods: This is a 12-month multicenter cohort study conducted in Italy. The primary outcomes included the description of PA by type and intensity, adherence to World Health Organization guidelines, bleeding, and health-related quality of life by EQ-5D questionnaire. PA data were collected continuously through a fitness tracker worn by the patient; all the other variables were collected through ePRO questionnaires. Results: Only 54 of the 103 enrolled subjects (52.4%) used their fitness tracker for the defined valid period; adolescents were the least compliant age group. PA was performed at low rates and intensity. Approximately 52% of the subjects had sedentary behavior. The mean EQ-5D values did not change over time. At least one bleeding was reported in 43.7% of the subjects, mostly with sedentary behavior. The PA in the 2 days preceding the bleeding was comparable to the one observed in the overall observational period. Conclusions: The systematic recording of data through a fitness tracker and ePRO app shows that subjects with HA without inhibitors have lower-than-expected PA and that they still experience issues related to bleeding.
Keywords: activity; bleeding; cohort studies; congenital; factor VIII deficiency; hemophilia A; pain; patient outcome; physical; quality of life.
Conflict of interest statement
M.M.E., B.A., P.D. and G.S. declare no conflicts of interest; B.C. declares that she has received consulting fees from Bayer and Novo Nordisk; M.R. declares that he has received consulting fees, contracts, and payment honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Roche, Bayer, Sobi, Kedrion, Takeda and Novo Nordisk; CG declares that he has received consulting fees from Uniqure, Biomarin, Roche, CSL Behring, and payment honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bayer, Takeda, SOBI, Roche, Kedrion, LFB, Novo Nordisk, Sanofi, CSL Behring, Uniqure, Werfen; S.L., T.R., B.S. and P.E. are full-time employee at Roche Italia.
Figures







References
LinkOut - more resources
Full Text Sources

