HER2-Altered Non-Small Cell Lung Cancer: A Journey from Current Approaches to Emerging Strategies
- PMID: 38893138
- PMCID: PMC11171190
- DOI: 10.3390/cancers16112018
HER2-Altered Non-Small Cell Lung Cancer: A Journey from Current Approaches to Emerging Strategies
Abstract
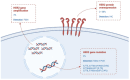
For patients diagnosed with advanced HER2-altered non-small cell lung cancer (NSCLC), the current standard of care is represented by a platinum-pemetrexed-based chemotherapy, eventually in combination with immunotherapy. Different pan-HER tyrosine kinase inhibitors have been evaluated in limited phase II trials, yielding generally unsatisfactory outcomes, although certain genotypes demonstrated some clinical benefit. Conversely, antibody-drug conjugates (ADCs) targeting HER2, particularly trastuzumab-deruxtecan, have shown promising results against HER2-mutant disease, including a great intracranial activity in patients with brain metastasis. Based on the results obtained from DESTINY-Lung01 and DESTINY-Lung02 trials, trastuzumab deruxtecan received regulatory approval as the first targeted therapy for pre-treated, HER2-mutant, advanced NSCLC patients. More recently, the Food and Drug Administration (FDA) granted the accelerated approval of trastuzumab deruxtecan for advanced, pre-treated HER2-positive solid tumours with no other treatment options. In this scenario, emerging evidence is increasingly pointing towards the exploration of combination regimens with synergistic effects in the advanced disease. In this review, we provide a detailed summary of current approaches and emerging strategies in the management of HER2-altered NSCLC, also focusing on unmet needs, including the treatment of patients with brain metastases.
Keywords: HER2; antibody-drug conjugates; non-small cell lung cancer; targeted therapy.
Conflict of interest statement
F.P. declared consultant/advisory fees from Astra Zeneca, Janssen, Sanofi, Amgen, Roche, Bristol Myer Squibb, Beigene, and Thermofisher Scientific. S.N. declared speaker bureau/advisor’s fees from Boehringer Ingelheim, Roche, Merck Sharp, Dohme, Amgen, Thermo Fisher Scientific, Eli Lilly, GlaxoSmithKline, Merck, AstraZeneca, Janssen, Novartis, Takeda, Bayer, Pfizer. The other authors have no conflicts of interest to declare.
Figures
References
-
- Pahuja K.B., Nguyen T.T., Jaiswal B.S., Prabhash K., Thaker T.M., Senger K., Chaudhuri S., Kljavin N.M., Antony A., Phalke S., et al. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell. 2018;34:792–806.e5. doi: 10.1016/J.CCELL.2018.09.010. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous