N-Benzylated 5-Hydroxybenzothiophene-2-carboxamides as Multi-Targeted Clk/Dyrk Inhibitors and Potential Anticancer Agents
- PMID: 38893153
- PMCID: PMC11171218
- DOI: 10.3390/cancers16112033
N-Benzylated 5-Hydroxybenzothiophene-2-carboxamides as Multi-Targeted Clk/Dyrk Inhibitors and Potential Anticancer Agents
Abstract
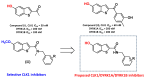
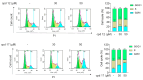
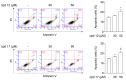
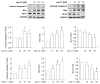
Numerous studies have reported that Dyrk1A, Dyrk1B, and Clk1 are overexpressed in multiple cancers, suggesting a role in malignant disease. Here, we introduce a novel class of group-selective kinase inhibitors targeting Dyrk1A, Dyrk1B, and Clk1. This was achieved by modifying our earlier selective Clk1 inhibitors, which were based on the 5-methoxybenzothiophene-2-carboxamide scaffold. By incorporating a 5-hydroxy group, we increased the potential for additional hydrogen bond interactions that broadened the inhibitory effect to include Dyrk1A and Dyrk1B kinases. Within this series, compounds 12 and 17 emerged as the most potent multi-kinase inhibitors against Dyrk1A, Dyrk1B, and Clk1. Furthermore, when assessed against the most closely related kinases also implicated in cancer, the frontrunner compounds revealed additional inhibitory activity against Haspin and Clk2. Compounds 12 and 17 displayed high potency across various cancer cell lines with minimal effect on non-tumor cells. By examining the effect of these inhibitors on cell cycle distribution, compound 17 retained cells in the G2/M phase and induced apoptosis. Compounds 12 and 17 could also increase levels of cleaved caspase-3 and Bax, while decreasing the expression of the antiapoptotic Bcl-2 protein. These findings support the further study and development of these compounds as novel anticancer therapeutics.
Keywords: 5-hyrdoxybenzothiophene; Clk1; Dyrk1A; apoptosis induction; cell cycle analysis; multi-targeting.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

