Ponatinib as a Prophylactic or Pre-Emptive Strategy to Prevent Cytological Relapse after Allogeneic Stem Cell Transplantation in Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Transplanted in Complete Cytological Remission
- PMID: 38893226
- PMCID: PMC11171293
- DOI: 10.3390/cancers16112108
Ponatinib as a Prophylactic or Pre-Emptive Strategy to Prevent Cytological Relapse after Allogeneic Stem Cell Transplantation in Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Transplanted in Complete Cytological Remission
Abstract
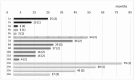
The administration of TKIs after Allo-SCT in Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL) remains controversial, and the TKI approach (prophylactic, pre-emptive or salvage) is still heterogeneous in transplant centers. In this context, very little is known about the feasibility and safety of third-generation TKIs. In this paper, we analyze the efficacy and safety of ponatinib (PONA) administered after Allo-SCT to prevent cytologic relapse of Ph + ALL. This is a multicenter observational study including 48 patients (pts) with Ph + ALL (median age 49 years) who received PONA after Allo-SCT while in complete cytological remission (cCR); 26 (54%) had positive minimal residual disease (MRD pos) before Allo-SCT. PONA was administered after Allo-SCT prophylactically (starting with MRD neg) in 26 pts or pre-emptively (starting with MRD pos post-SCT and without hematological relapse) in 22 pts. Patients treated prophylactically with PONA started treatment earlier, at a median of 4.3 months (range 1.5-6) after Allo-SCT, than those treated pre-emptively, who started PONA at a median of 7.4 months (range 2-63) after Allo-SCT (p = 0.01). The median starting dose of PONA was 30 mg/day (range 15-45). A dose reduction was required in 10/48 (21%) of cases, but a permanent discontinuation of PONA, due to toxicity, was required in only 5/48 pts (10.5%). No deaths due to PONA-related adverse events (AEs) were reported. The median follow-up time after Allo-SCT was 34 months (range 7.7-118). At the last follow-up, the median duration of PONA therapy was 22 months (range 2-100). The 5-year OS and RFS after Allo-SCT were 92% and 71%, respectively. The 5-year RFS after Allo-SCT of pts who received PONA prophylaxis was 95%, and it was 57% for those who received PONA pre-emptively (log-rank p = 0.02). In conclusion, this multicenter analysis of 48 patients with Ph + ALL undergoing Allo-SCT while in CcR, although with the caution of the retrospective data, supports the feasibility of PONA maintenance strategy after Allo-SCT with a low rate of discontinuations (10.5%) due to PONA-related AE.
Keywords: TKI; acute lymphoblastic leukemia; allogeneic stem cell transplantation; ponatinib.
Conflict of interest statement
The authors declare no conflicts of interest related to the submitted study.
Figures


References
-
- Gökbuget N., Boissel N., Chiaretti S., Dombret H., Doubek M., Fielding A.K., Foà R., Giebel S., Hoelzer D., Hunault M., et al. Diagnosis, Prognostic Factors and Assessment of ALL in Adults: 2023 ELN Recommendations from a European Expert Panel. Blood. 2024;143:1891–1902. doi: 10.1182/blood.2023020794. - DOI - PubMed
-
- Bazarbachi A., Labopin M., Aljurf M., Niittyvuopio R., Balsat M., Blaise D., Yakoub-Agha I., Grassi A., Reinhardt H.C., Lenhoff S., et al. 20-year steady increase in survival of adult patients with relapsed philadelphia-positive acute lymphoblastic leukemia post allogeneic hematopoietic cell transplantation. Clin. Cancer Res. 2022;28:1004–1012. doi: 10.1158/1078-0432.Ccr-21-2675. - DOI - PubMed
LinkOut - more resources
Full Text Sources

