Flow Cytometry Profiling of Plasmacytoid Dendritic Cell Neoplasms
- PMID: 38893237
- PMCID: PMC11171351
- DOI: 10.3390/cancers16112118
Flow Cytometry Profiling of Plasmacytoid Dendritic Cell Neoplasms
Abstract
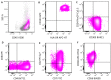
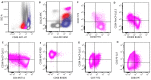
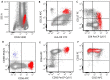
In this review, we aim to provide a summary of the diverse immunophenotypic presentations of distinct entities associated with plasmacytoid dendritic cell (pDC) proliferation. These entities include the following: (1) blastic plasmacytoid dendritic cell neoplasm (BPDCN); (2) mature pDC proliferation (MPDCP), most commonly seen in chronic myelomonocytic leukemia (CMML); and (3) myeloid neoplasms with pDC differentiation, in which pDCs show a spectrum of maturation from early immature pDCs to mature forms, most commonly seen in acute myeloid leukemia (pDC-AML). Our aim is to provide a flow cytometry diagnostic approach to these distinct and sometimes challenging entities and to clarify the immunophenotypic spectrum of neoplastic pDCs in different disease presentations. In this review, we also cover the strategies in the evaluation of residual disease, as well as the challenges and pitfalls we face in the setting of immune and targeted therapy. The differential diagnosis will also be discussed, as blasts in some AML cases can have a pDC-like immunophenotype, mimicking pDCs.
Keywords: AML with plasmacytoid dendritic cell differentiation; BPDCN; MPDCP; blastic plasmacytoid dendritic cell neoplasm; flow cytometry analysis; mature plasmacytoid dendritic cell proliferation; pDC-AML; plasmacytoid dendritic cells.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures

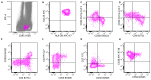
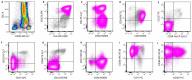
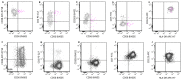
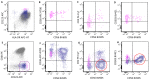
Similar articles
-
Diagnostic approach to blastic plasmacytoid dendritic cell neoplasm: historical perspectives and current understanding.J Clin Exp Hematop. 2025;65(1):1-16. doi: 10.3960/jslrt.24069. J Clin Exp Hematop. 2025. PMID: 40159280 Free PMC article. Review.
-
Immunophenotypic and Molecular Features of Acute Myeloid Leukemia with Plasmacytoid Dendritic Cell Differentiation Are Distinct from Blastic Plasmacytoid Dendritic Cell Neoplasm.Cancers (Basel). 2022 Jul 11;14(14):3375. doi: 10.3390/cancers14143375. Cancers (Basel). 2022. PMID: 35884435 Free PMC article.
-
Myeloid Neoplasms with Elevated Plasmacytoid Dendritic Cell Differentiation Reflect the Maturation Process of Dendritic Cells.Cytometry A. 2020 Jan;97(1):61-69. doi: 10.1002/cyto.a.23953. Epub 2019 Dec 26. Cytometry A. 2020. PMID: 31876105
-
Plasmacytoid dendritic cells in the setting of myeloid neoplasms: Diagnostic guide to challenging pathologic presentations.Br J Haematol. 2023 Mar;200(5):545-555. doi: 10.1111/bjh.18632. Epub 2023 Jan 6. Br J Haematol. 2023. PMID: 36606610 Review.
-
Plasmacytoid Dendritic Cells, a Novel Target in Myeloid Neoplasms.Cancers (Basel). 2022 Jul 21;14(14):3545. doi: 10.3390/cancers14143545. Cancers (Basel). 2022. PMID: 35884612 Free PMC article. Review.
Cited by
-
Diagnostic approach to blastic plasmacytoid dendritic cell neoplasm: historical perspectives and current understanding.J Clin Exp Hematop. 2025;65(1):1-16. doi: 10.3960/jslrt.24069. J Clin Exp Hematop. 2025. PMID: 40159280 Free PMC article. Review.
-
Association Between B-Cell Marker Expression and RUNX1 Lesions in Acute Myeloid Leukemia, Beyond RUNX1::RUNX1T1 Fusion: Diagnostic Pitfalls with Mixed-Phenotype Acute Leukemia-B/Myeloid.Cancers (Basel). 2025 Apr 18;17(8):1354. doi: 10.3390/cancers17081354. Cancers (Basel). 2025. PMID: 40282530 Free PMC article.
-
BPDCN: state of the art.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):279-286. doi: 10.1182/hematology.2024000553. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39644068 Free PMC article. Review.
-
Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm.Int J Mol Sci. 2025 Mar 18;26(6):2732. doi: 10.3390/ijms26062732. Int J Mol Sci. 2025. PMID: 40141368 Free PMC article. Review.
References
-
- Lucas N., Duchmann M., Rameau P., Noël F., Michea P., Saada V., Kosmider O., Pierron G., Fernandez-Zapico M.E., Howard M.T., et al. Biology and prognostic impact of clonal plasmacytoid dendritic cells in chronic myelomonocytic leukemia. Leukemia. 2019;33:2466–2480. doi: 10.1038/s41375-019-0447-3. - DOI - PubMed
-
- Wang W., Khoury J.D., Miranda R.N., Jorgensen J.L., Xu J., Loghavi S., Li S., Pemmaraju N., Nguyen T., Medeiros L.J., et al. Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a 10-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm. Haematologica. 2021;106:1047–1055. doi: 10.3324/haematol.2020.247569. - DOI - PMC - PubMed
-
- Xiao W., Goldberg A.D., Famulare C.A., Devlin S.M., Nguyen N.T., Sim S., Kabel C.C., Patel M.A., McGovern E.M., Patel A., et al. Loss of plasmacytoid dendritic cell differentiation is highly predictive for post-induction measurable residual disease and inferior outcomes in acute myeloid leukemia. Haematologica. 2019;104:1378–1387. doi: 10.3324/haematol.2018.203018. - DOI - PMC - PubMed
-
- Murthy H.S., Zhang M.-J., Chen K., Ahmed S., Deotare U., Ganguly S., Kansagra A., Michelis F.V., Nishihori T., Patnaik M., et al. Allogeneic hematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: A CIBMTR analysis. Blood Adv. 2023;7:7007–7016. doi: 10.1182/bloodadvances.2023011308. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

