Advances in Nucleic Acid Assays for Infectious Disease: The Role of Microfluidic Technology
- PMID: 38893293
- PMCID: PMC11173870
- DOI: 10.3390/molecules29112417
Advances in Nucleic Acid Assays for Infectious Disease: The Role of Microfluidic Technology
Abstract
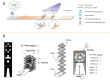
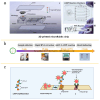
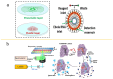
Within the fields of infectious disease diagnostics, microfluidic-based integrated technology systems have become a vital technology in enhancing the rapidity, accuracy, and portability of pathogen detection. These systems synergize microfluidic techniques with advanced molecular biology methods, including reverse transcription polymerase chain reaction (RT-PCR), loop-mediated isothermal amplification (LAMP), and clustered regularly interspaced short palindromic repeats (CRISPR), have been successfully used to identify a diverse array of pathogens, including COVID-19, Ebola, Zika, and dengue fever. This review outlines the advances in pathogen detection, attributing them to the integration of microfluidic technology with traditional molecular biology methods and smartphone- and paper-based diagnostic assays. The cutting-edge diagnostic technologies are of critical importance for disease prevention and epidemic surveillance. Looking ahead, research is expected to focus on increasing detection sensitivity, streamlining testing processes, reducing costs, and enhancing the capability for remote data sharing. These improvements aim to achieve broader coverage and quicker response mechanisms, thereby constructing a more robust defense for global public health security.
Keywords: high throughput; infectious disease; microfluidic system; nucleic acid assay; rapid diagnosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Santiago G.A., Vázquez J., Courtney S., Matías K.Y., Andersen L.E., Colón C., Butler A.E., Roulo R., Bowzard J., Villanueva J.M., et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat. Commun. 2018;9:1391. doi: 10.1038/s41467-018-03772-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
- No.2021YFF1200800/the National Key Research and Development Program of China
- 22140900900/the Science and Technology Commission of Shanghai Municipality
- 23141901300/the Science and Technology Commission of Shanghai Municipality
- 22xtcx00100/the Science and Technology Commission of Shanghai Municipality
- XTCX-KJ-2022-32/the Science and Technology Commission of Shanghai Municipality
LinkOut - more resources
Full Text Sources

