A Novel Electrochemical Sensor Based on Pd Confined Mesoporous Carbon Hollow Nanospheres for the Sensitive Detection of Ascorbic Acid, Dopamine, and Uric Acid
- PMID: 38893303
- PMCID: PMC11173461
- DOI: 10.3390/molecules29112427
A Novel Electrochemical Sensor Based on Pd Confined Mesoporous Carbon Hollow Nanospheres for the Sensitive Detection of Ascorbic Acid, Dopamine, and Uric Acid
Abstract
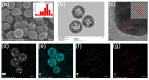
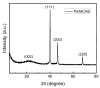
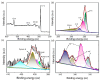
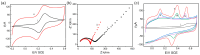
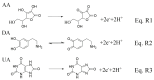
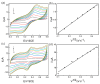
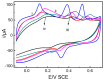
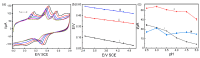
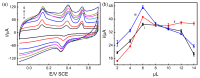
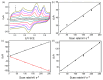
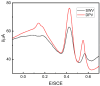
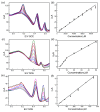
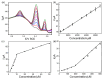
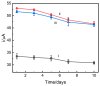
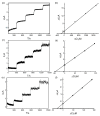
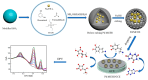
In this study, we designed a novel electrochemical sensor by modifying a glass carbon electrode (GCE) with Pd confined mesoporous carbon hollow nanospheres (Pd/MCHS) for the simultaneous detection of ascorbic acid (AA), dopamine (DA), and uric acid (UA). The structure and morphological characteristics of the Pd/MCHS nanocomposite and the Pd/MCHS/GCE sensor are comprehensively examined using SEM, TEM, XRD and EDX. The electrochemical properties of the prepared sensor are investigated through CV and DPV, which reveal three resolved oxidation peaks for AA, DA, and UA, thereby verifying the simultaneous detection of the three analytes. Benefiting from its tailorable properties, the Pd/MCHS nanocomposite provides a large surface area, rapid electron transfer ability, good catalytic activity, and high conductivity with good electrochemical behavior for the determination of AA, DA, and UA. Under optimized conditions, the Pd/MCHS/GCE sensor exhibited a linear response in the concentration ranges of 300-9000, 2-50, and 20-500 µM for AA, DA, and UA, respectively. The corresponding limit of detection (LOD) values were determined to be 51.03, 0.14, and 4.96 µM, respectively. Moreover, the Pd/MCHS/GCE sensor demonstrated outstanding selectivity, reproducibility, and stability. The recovery percentages of AA, DA, and UA in real samples, including a vitamin C tablet, DA injection, and human urine, range from 99.8-110.9%, 99.04-100.45%, and 98.80-100.49%, respectively. Overall, the proposed sensor can serve as a useful reference for the construction of a high-performance electrochemical sensing platform.
Keywords: Pd/MCHS; and uric acid; ascorbic acid; dopamine; electrochemical sensor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor.Anal Biochem. 2010 May 1;400(1):78-88. doi: 10.1016/j.ab.2010.01.001. Epub 2010 Jan 11. Anal Biochem. 2010. PMID: 20064483
-
Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on graphene anchored with Pd-Pt nanoparticles.Colloids Surf B Biointerfaces. 2013 Nov 1;111:392-7. doi: 10.1016/j.colsurfb.2013.06.030. Epub 2013 Jun 21. Colloids Surf B Biointerfaces. 2013. PMID: 23850748
-
Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite.J Nanobiotechnology. 2020 Aug 10;18(1):112. doi: 10.1186/s12951-020-00672-9. J Nanobiotechnology. 2020. PMID: 32778119 Free PMC article.
-
Recent Advances in MOF-Based Materials for Biosensing Applications.Sensors (Basel). 2025 Apr 14;25(8):2473. doi: 10.3390/s25082473. Sensors (Basel). 2025. PMID: 40285162 Free PMC article. Review.
-
Electrochemical biosensors for dopamine.Clin Chim Acta. 2025 Jan 30;566:120039. doi: 10.1016/j.cca.2024.120039. Epub 2024 Nov 15. Clin Chim Acta. 2025. PMID: 39550057 Review.
Cited by
-
Copper Nanoclusters Anchored on Crumpled N-Doped MXene for Ultra-Sensitive Electrochemical Sensing.Sensors (Basel). 2025 Apr 16;25(8):2508. doi: 10.3390/s25082508. Sensors (Basel). 2025. PMID: 40285194 Free PMC article.
References
-
- Yang L., Wang A.-J., Weng X., Feng J.-J. Well-dispersed strawberry-like PtCo nanocrystals/porous N-doped carbon nanospheres for multiplexed assays. Microchem. J. 2023;187:108421. doi: 10.1016/j.microc.2023.108421. - DOI
-
- Wu P., Huang Y., Zhao X., Lin D., Xie L., Li Z., Zhu Z., Zhao H., Lan M. MnFe2O4/MoS2 nanocomposite as Oxidase-like for electrochemical simultaneous detection of ascorbic acid, dopamine and uric acid. Microchem. J. 2022;181:107780. doi: 10.1016/j.microc.2022.107780. - DOI
-
- Ma C., Xu P., Chen H., Cui J., Guo M., Zhao J. An electrochemical sensor based on reduced graphene oxide/β-cyclodextrin/multiwall carbon nanotubes/polyoxometalate tetracomponent hybrid: Simultaneous determination of ascorbic acid, dopamine and uric acid. Microchem. J. 2022;180:107533. doi: 10.1016/j.microc.2022.107533. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

