Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine
- PMID: 38893309
- PMCID: PMC11173670
- DOI: 10.3390/molecules29112433
Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine
Abstract
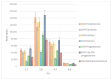
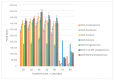
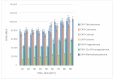
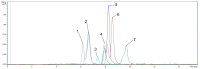
The possibility of cyanoacetohydrazide usage as a novel derivatizing agent is demonstrated in the presented article, and a comparison with hydroxylamine as the most commonly used reagent is provided. Optimal conditions for steroid derivatization with cyanoacetohydrazide are provided. According to the collected data, the maximum yield of derivatives was observed at pH 2.8 within 70 min at 40 °C with 5 ng/mL limit of detection for all investigated analytes. It was shown that cyanoacetohydrazide derivatives produces both syn- and anti-forms as well as hydroxylamine, and their ratios were evaluated and shown in presented work. An efficiency enchantment from two to up to five times was achieved with a novel derivatization reagent. Its applicability for qualitative analysis of steroids in urine was presented at real samples. Additionally, the reproducible fragmentation of the derivatizing agent in collision-induced dissociation offers opportunities for simplified non-targeted steroidomic screening. Furthermore, cyanoacetohydrazide increases ionization efficiency in positive mode, which can eliminate the need for redundant high-resolution instrument runs required for both positive and negative mode analyses.
Keywords: LC-HRMS; cyanoacetohydrazide; derivatization; non-targeted screening; steroidomics; steroids; testosterone.
Conflict of interest statement
Author Sanka N. Atapattu was employed by the company CanAm Bioresearch Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Solid-phase analytical derivatization as a tool for the quantification of steroid hormones in human urine with HPLC-Q-ToF detection.J Pharm Biomed Anal. 2022 May 30;214:114736. doi: 10.1016/j.jpba.2022.114736. Epub 2022 Mar 21. J Pharm Biomed Anal. 2022. PMID: 35338944
-
Improving liquid chromatography-tandem mass spectrometry determination of polycarboxylic acids in human urine by chemical derivatization. Comparison of o-benzyl hydroxylamine and 2-picolyl amine.J Pharm Biomed Anal. 2019 Feb 5;164:382-394. doi: 10.1016/j.jpba.2018.10.055. Epub 2018 Nov 3. J Pharm Biomed Anal. 2019. PMID: 30466023
-
Phthalylglycyl Chloride as a Derivatization Agent for UHPLC-MS/MS Determination of Adrenaline, Dopamine and Octopamine in Urine.Molecules. 2023 Mar 23;28(7):2900. doi: 10.3390/molecules28072900. Molecules. 2023. PMID: 37049663 Free PMC article.
-
Ultra high performance liquid chromatography tandem mass spectrometry determination and profiling of prohibited steroids in human biological matrices. A review.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 May 15;927:22-36. doi: 10.1016/j.jchromb.2012.12.003. Epub 2012 Dec 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 23317577 Review.
-
Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review.J Steroid Biochem Mol Biol. 2016 Sep;162:57-69. doi: 10.1016/j.jsbmb.2015.10.003. Epub 2015 Oct 17. J Steroid Biochem Mol Biol. 2016. PMID: 26454158 Review.
References
-
- Kalogera E., Pistos C., Provatopoulou X., Athanaselis S., Spiliopoulou C., Gounaris A. Androgen glucuronides analysis by liquid chromatography tandem-mass spectrometry: Could it raise new perspectives in the diagnostic field of hormone-dependent malignancies? J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013;940:24–34. doi: 10.1016/j.jchromb.2013.09.022. - DOI - PubMed
-
- Falk R.T., Xu X., Keefer L., Veenstra T.D., Zieger R.G. A Liquid Chromatography-Mass Spectrometry Method for the Simultaneous Measurement of Fifteen Urinary Estrogens and Estrogen Metabolites: Assay Reproducibility and Inter-individual Variability. Cancer Epidemiol. Biomark. Prev. 2008;17:3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

