Strategies for Accessing cis-1-Amino-2-Indanol
- PMID: 38893318
- PMCID: PMC11173559
- DOI: 10.3390/molecules29112442
Strategies for Accessing cis-1-Amino-2-Indanol
Abstract
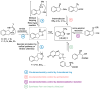
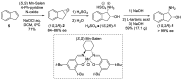
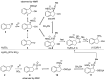
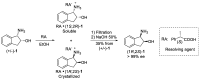
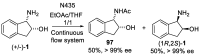
cis-1-amino-2-indanol is an important building block in many areas of chemistry. Indeed, this molecule is currently used as skeleton in many ligands (BOX, PyBOX…), catalysts and chiral auxiliaries. Moreover, it has been incorporated in numerous bioactive structures. The major issues during its synthesis are the control of cis-selectivity, for which various strategies have been devised, and the enantioselectivity of the reaction. This review highlights the various methodologies implemented over the last few decades to access cis-1-amino-2-indanol in racemic and enantioselective manners. In addition, the various substitution patterns on the aromatic ring and their preparations are listed.
Keywords: C-H activation; Ritter reaction; chemical resolution; cis-1-amino-2-indanol; enantioselective reaction; enzymatic resolution; epimerization; intramolecular cyclization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

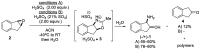
















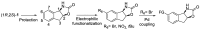
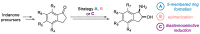
Similar articles
-
Silylative Kinetic Resolution of Racemic 1-Indanol Derivatives Catalyzed by Chiral Guanidine.J Org Chem. 2018 Jan 5;83(1):452-458. doi: 10.1021/acs.joc.7b02493. Epub 2017 Dec 21. J Org Chem. 2018. PMID: 29231721
-
New chiral ruthenium(II) catalysts containing 2,6-bis(4'-(R)-phenyloxazolin-2'-yl)pyridine (Ph-pybox) ligands for highly enantioselective transfer hydrogenation of ketones.Chemistry. 2004 Jan 23;10(2):425-32. doi: 10.1002/chem.200305170. Chemistry. 2004. PMID: 14735511
-
Applications of Iridium-Catalyzed Asymmetric Allylic Substitution Reactions in Target-Oriented Synthesis.Acc Chem Res. 2017 Oct 17;50(10):2539-2555. doi: 10.1021/acs.accounts.7b00300. Epub 2017 Sep 22. Acc Chem Res. 2017. PMID: 28937739
-
Catalytic asymmetric organozinc additions to carbonyl compounds.Chem Rev. 2001 Mar;101(3):757-824. doi: 10.1021/cr000411y. Chem Rev. 2001. PMID: 11712502 Review.
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
References
-
- Saddique F.A., Zahoor A.F., Faiz S., Naqvi A.A.R., Usman M., Ahmad M. Recent trends in ring opening of epoxides by amines as nucleophiles. Synth. Commun. 2016;46:831–868. doi: 10.1080/00397911.2016.1170148. - DOI
-
- Weng C., Zhang H., Xiong X., Lu X., Zhou Y. Evolution of Epoxides to Synthesize β-amino Alcohols. Asian J. Org. Chem. 2014;26:3761–3768. doi: 10.14233/ajchem.2014.16015. - DOI
-
- Bergmeier S.C. The Synthesis of Vicinal Amino Alcohols. Tetrahedron. 2000;56:2561–2576. doi: 10.1016/S0040-4020(00)00149-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources

