Synthesis of Thiazolidin-4-Ones Derivatives, Evaluation of Conformation in Solution, Theoretical Isomerization Reaction Paths and Discovery of Potential Biological Targets
- PMID: 38893334
- PMCID: PMC11173912
- DOI: 10.3390/molecules29112458
Synthesis of Thiazolidin-4-Ones Derivatives, Evaluation of Conformation in Solution, Theoretical Isomerization Reaction Paths and Discovery of Potential Biological Targets
Abstract
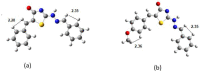
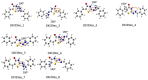
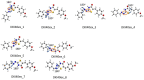
Thiazolin-4-ones and their derivatives represent important heterocyclic scaffolds with various applications in medicinal chemistry. For that reason, the synthesis of two 5-substituted thiazolidin-4-one derivatives was performed. Their structure assignment was conducted by NMR experiments (2D-COSY, 2D-NOESY, 2D-HSQC and 2D-HMBC) and conformational analysis was conducted through Density Functional Theory calculations and 2D-NOESY. Conformational analysis showed that these two molecules adopt exo conformation. Their global minimum structures have two double bonds (C=N, C=C) in Z conformation and the third double (C=N) in E. Our DFT results are in agreement with the 2D-NMR measurements. Furthermore, the reaction isomerization paths were studied via DFT to check the stability of the conformers. Finally, some potential targets were found through the SwissADME platform and docking experiments were performed. Both compounds bind strongly to five macromolecules (triazoloquinazolines, mglur3, Jak3, Danio rerio HDAC6 CD2, acetylcholinesterase) and via SwissADME it was found that these two molecules obey Lipinski's Rule of Five.
Keywords: DFT; NMR; drug-likeness; molecular docking; molecular dynamics; thazoline.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


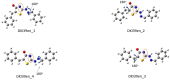











References
-
- Thakral S., Saini D., Kumar A., Jain N., Jain S. A Synthetic Approach and Molecular Docking Study of Hybrids of Quinazolin-4-Ones and Thiazolidin-4-Ones as Anticancer Agents. Med. Chem. Res. 2017;26:1595–1604. doi: 10.1007/s00044-017-1857-2. - DOI
-
- Shawky A.M., Abourehab M.A.S., Abdalla A.N., Gouda A.M. Optimization of Pyrrolizine-Based Schiff Bases with 4-Thiazolidinone Motif: Design, Synthesis and Investigation of Cytotoxicity and Anti-Inflammatory Potency. Eur. J. Med. Chem. 2020;185:111780. doi: 10.1016/j.ejmech.2019.111780. - DOI - PubMed
-
- Patel A.D., Pasha T.Y., Lunagariya P., Shah U., Bhambharoliya T., Tripathi R.K.P. A Library of Thiazolidin-4-one Derivatives as Protein Tyrosine Phosphatase 1B (PTP1B) Inhibit: An Attempt To Discover Novel Antidiabetic Agents. ChemMedChem. 2020;15:1229–1242. doi: 10.1002/cmdc.202000055. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

