The Application of Ultrasmall Gold Nanoparticles (2 nm) Functionalized with Doxorubicin in Three-Dimensional Normal and Glioblastoma Organoid Models of the Blood-Brain Barrier
- PMID: 38893345
- PMCID: PMC11173746
- DOI: 10.3390/molecules29112469
The Application of Ultrasmall Gold Nanoparticles (2 nm) Functionalized with Doxorubicin in Three-Dimensional Normal and Glioblastoma Organoid Models of the Blood-Brain Barrier
Abstract
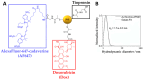
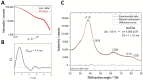
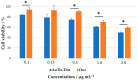
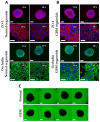
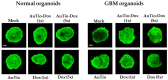
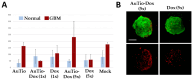
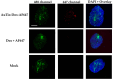
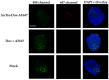
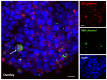
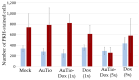
Among brain tumors, glioblastoma (GBM) is very challenging to treat as chemotherapeutic drugs can only penetrate the brain to a limited extent due to the blood-brain barrier (BBB). Nanoparticles can be an attractive solution for the treatment of GBM as they can transport drugs across the BBB into the tumor. In this study, normal and GBM organoids comprising six brain cell types were developed and applied to study the uptake, BBB penetration, distribution, and efficacy of fluorescent, ultrasmall gold nanoparticles (AuTio-Dox-AF647s) conjugated with doxorubicin (Dox) and AlexaFluor-647-cadaverine (AF647) by confocal laser scanning microscopy (CLSM), using a mixture of dissolved doxorubicin and fluorescent AF647 molecules as a control. It was shown that the nanoparticles could easily penetrate the BBB and were found in normal and GBM organoids, while the dissolved Dox and AF647 molecules alone were unable to penetrate the BBB. Flow cytometry showed a reduction in glioblastoma cells after treatment with AuTio-Dox nanoparticles, as well as a higher uptake of these nanoparticles by GBM cells in the GBM model compared to astrocytes in the normal cell organoids. In summary, our results show that ultrasmall gold nanoparticles can serve as suitable carriers for the delivery of drugs into organoids to study BBB function.
Keywords: blood–brain barrier; doxorubicin; drug delivery; glioblastoma; gold; nanoparticles; organoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

