Design, Synthesis, and Photophysical Properties of 5-Aminobiphenyl Substituted [1,2,4]Triazolo[4,3- c]- and [1,2,4]Triazolo[1,5- c]quinazolines
- PMID: 38893371
- PMCID: PMC11173969
- DOI: 10.3390/molecules29112497
Design, Synthesis, and Photophysical Properties of 5-Aminobiphenyl Substituted [1,2,4]Triazolo[4,3- c]- and [1,2,4]Triazolo[1,5- c]quinazolines
Abstract
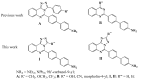
Two series of novel [1,2,4]triazolo[4,3-c]- and [1,2,4]triazolo[1,5-c]quinazoline fluorophores with 4'-amino[1,1']-biphenyl residue at position 5 have been prepared via Pd-catalyzed cross-coupling Suzuki-Miyaura reactions. The treatment of 2-(4-bromophenyl)-4-hydrazinoquinazoline with orthoesters in solvent-free conditions or in absolute ethanol leads to the formation of [4,3-c]-annulated triazoloquinazolines, whereas [1,5-c] isomers are formed in acidic media as a result of Dimroth rearrangement. A 1D-NMR and 2D-NMR spectroscopy, as well as a single-crystal X-ray diffraction analysis, unambiguously confirmed the annelation type and determined the molecular structure of p-bromophenyl intermediates and target products. Photophysical properties of the target compounds were investigated in two solvents and in the solid state and compared with those of related 3-aryl-substituted [1,2,4]triazolo[4,3-c]quinazolines. The exclusion of the aryl fragment from the triazole ring has been revealed to improve fluorescence quantum yield in solution. Most of the synthesized structures show moderate to high quantum yields in solution. Additionally, the effect of solvent polarity on the absorption and emission spectra of fluorophores has been studied, and considerable fluorosolvatochromism has been stated. Moreover, electrochemical investigation and DFT calculations have been performed; their results are consistent with the experimental observation.
Keywords: [1,2,4]triazolo[1,5-c]quinazoline; [1,2,4]triazolo[4,3-c]quinazoline; cross-coupling; fluorescence; solvatochromism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Jabeen T., Aslam S., Ahmad M., ul Haq A., Al-Hussain S.A., Zaki M.E.A. Recent Advances on Quinazoline [Working Title] IntechOpen; London, UK: 2023. Triazoloquinazoline: Synthetic Strategies and Medicinal Importance.
-
- Motoyama M., Doan T.H., Hibner-Kulicka P., Otake R., Lukarska M., Lohier J.F., Ozawa K., Nanbu S., Alayrac C., Suzuki Y., et al. Synthesis and Structure-Photophysics Evaluation of 2-N-Amino-Quinazolines: Small Molecule Fluorophores for Solution and Solid State. Chem. Asian J. 2021;16:2087–2099. doi: 10.1002/asia.202100534. - DOI - PubMed
-
- Mao M., Zhang X., Zhu B., Wang J., Wu G., Yin Y., Song Q. Comparative Studies of Organic Dyes with a Quinazoline or Quinoline Chromophore as π-Conjugated Bridges for Dye-Sensitized Solar Cells. Dye. Pigment. 2016;124:72–81. doi: 10.1016/j.dyepig.2015.09.002. - DOI
-
- Bonnaud T., Scaviner M., Robin-le Guen F., Achelle S. 4-substituted Push-pull Quinazoline Chromophores with Extended π-conjugated Linker. J. Heterocycl. Chem. 2024;61:358–364. doi: 10.1002/jhet.4768. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

