Carbohydrate-Binding Properties and Antimicrobial and Anticancer Potential of a New Lectin from the Phloem Sap of Cucurbita pepo
- PMID: 38893406
- PMCID: PMC11174025
- DOI: 10.3390/molecules29112531
Carbohydrate-Binding Properties and Antimicrobial and Anticancer Potential of a New Lectin from the Phloem Sap of Cucurbita pepo
Abstract
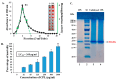
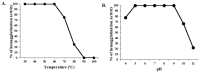
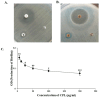
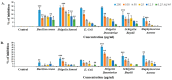
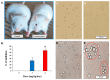
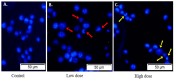
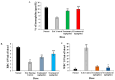
A Cucurbita phloem exudate lectin (CPL) from summer squash (Cucurbita pepo) fruits was isolated and its sugar-binding properties and biological activities were studied. The lectin was purified by affinity chromatography and the hemagglutination assay method was used to determine its pH, heat stability, metal-dependency and sugar specificity. Antimicrobial and anticancer activities were also studied by disc diffusion assays and in vivo and in vitro methods. The molecular weight of CPL was 30 ± 1 KDa and it was stable at different pH (5.0 to 9.0) and temperatures (30 to 60 °C). CPL recovered its hemagglutination activity in the presence of Ca2+. 4-nitrophenyl-α-D-glucopyranoside, lactose, rhamnose and N-acetyl-D-glucosamine strongly inhibited the activity. With an LC50 value of 265 µg/mL, CPL was moderately toxic and exhibited bacteriostatic, bactericidal and antibiofilm activities against different pathogenic bacteria. It also exhibited marked antifungal activity against Aspergillus niger and agglutinated A. flavus spores. In vivo antiproliferative activity against Ehrlich ascites carcinoma (EAC) cells in Swiss albino mice was observed when CPL exerted 36.44% and 66.66% growth inhibition at doses of 3.0 mg/kg/day and 6.0 mg/kg/day, respectively. A 12-day treatment by CPL could reverse their RBC and WBC counts as well as restore the hemoglobin percentage to normal levels. The MTT assay of CPL performed against human breast (MCF-7) and lung (A-549) cancer cell lines showed 29.53% and 18.30% of inhibitory activity at concentrations of 128 and 256 µg/mL, respectively.
Keywords: Cucurbita lectins; antibiofilm; antifungal; antitumor; bacteriostatic; chitin binding.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Brudner M., Karpel M., Lear C., Chen L., Yantosca L.M., Scully C., Sarraju A., Sokolovska A., Zariffard M.R., Eisen D.P., et al. Lectin-Dependent Enhancement of Ebola Virus Infection via Soluble and Transmembrane C-Type Lectin Receptors. PLoS ONE. 2013;8:e60838. doi: 10.1371/journal.pone.0060838. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

