Poly(2-(dimethylamino)ethyl methacrylate)-Grafted Amphiphilic Block Copolymer Micelles Co-Loaded with Quercetin and DNA
- PMID: 38893415
- PMCID: PMC11173910
- DOI: 10.3390/molecules29112540
Poly(2-(dimethylamino)ethyl methacrylate)-Grafted Amphiphilic Block Copolymer Micelles Co-Loaded with Quercetin and DNA
Abstract
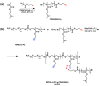
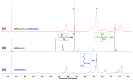
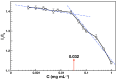
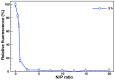
The synergistic effect of drug and gene delivery is expected to significantly improve cancer therapy. However, it is still challenging to design suitable nanocarriers that are able to load simultaneously anticancer drugs and nucleic acids due to their different physico-chemical properties. In the present work, an amphiphilic block copolymer comprising a biocompatible poly(ethylene glycol) (PEG) block and a multi-alkyne-functional biodegradable polycarbonate (PC) block was modified with a number of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) side chains applying the highly efficient azide-alkyne "click" chemistry reaction. The resulting cationic amphiphilic copolymer with block and graft architecture (MPEG-b-(PC-g-PDMAEMA)) self-associated in aqueous media into nanosized micelles which were loaded with the antioxidant, anti-inflammatory, and anticancer drug quercetin. The drug-loaded nanoparticles were further used to form micelleplexes in aqueous media through electrostatic interactions with DNA. The obtained nanoaggregates-empty and drug-loaded micelles as well as the micelleplexes intended for simultaneous DNA and drug codelivery-were physico-chemically characterized. Additionally, initial in vitro evaluations were performed, indicating the potential application of the novel polymer nanocarriers as drug delivery systems.
Keywords: DNA; click; codelivery; graft copolymer; metabolic activity; micelleplex; multifunctional; quercetin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Effects of the incorporation of a hydrophobic middle block into a PEG-polycation diblock copolymer on the physicochemical and cell interaction properties of the polymer-DNA complexes.Biomacromolecules. 2008 Nov;9(11):3294-307. doi: 10.1021/bm800876v. Epub 2008 Oct 23. Biomacromolecules. 2008. PMID: 18942877 Free PMC article.
-
Polycation Architecture and Assembly Direct Successful Gene Delivery: Micelleplexes Outperform Polyplexes via Optimal DNA Packaging.J Am Chem Soc. 2019 Oct 9;141(40):15804-15817. doi: 10.1021/jacs.9b06218. Epub 2019 Sep 25. J Am Chem Soc. 2019. PMID: 31553590
-
Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery.Biomaterials. 2009 Jun;30(16):3009-19. doi: 10.1016/j.biomaterials.2009.02.011. Epub 2009 Feb 27. Biomaterials. 2009. PMID: 19250665
-
Green Synthesis and the Evaluation of a Functional Amphiphilic Block Copolymer as a Micellar Curcumin Delivery System.Int J Mol Sci. 2023 Jun 24;24(13):10588. doi: 10.3390/ijms241310588. Int J Mol Sci. 2023. PMID: 37445767 Free PMC article.
-
Amphiphilic and biodegradable methoxy polyethylene glycol-block-(polycaprolactone-graft-poly(2-(dimethylamino)ethyl methacrylate)) as an effective gene carrier.Biomaterials. 2011 Jan;32(3):879-89. doi: 10.1016/j.biomaterials.2010.09.052. Epub 2010 Oct 20. Biomaterials. 2011. PMID: 20970186 Free PMC article.
Cited by
-
Review on various activator-assisted polymer grafting techniques for smart drug delivery applications.RSC Adv. 2025 Jul 4;15(28):23025-23044. doi: 10.1039/d5ra03188e. eCollection 2025 Jun 30. RSC Adv. 2025. PMID: 40621419 Free PMC article. Review.
References
-
- Palmiero U., Sponchioni M., Manfredini N., Maraldi M., Moscatelli D. Strategies to combine ROP with ATRP or RAFT polymerization for the synthesis of biodegradable polymeric nanoparticles for biomedical applications. Polym. Chem. 2018;9:4084–4099. doi: 10.1039/C8PY00649K. - DOI
-
- Krishnan A., Roy S., Menon S. Amphiphilic block copolymers: From synthesis including living polymerization methods to applications in drug delivery. Eur. Polym. J. 2022;172:111224. doi: 10.1016/j.eurpolymj.2022.111224. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

