Phenanthroline-Mediated Photoelectrical Enhancement in Calix[4]arene-Functionalized Titanium-Oxo Clusters
- PMID: 38893442
- PMCID: PMC11173645
- DOI: 10.3390/molecules29112566
Phenanthroline-Mediated Photoelectrical Enhancement in Calix[4]arene-Functionalized Titanium-Oxo Clusters
Abstract
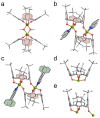
Incorporating two organic ligands with different functionalities into a titanium-oxo cluster entity simultaneously can endow the material with their respective properties and provide synergistic performance enhancement, which is of great significance for enriching the structure and properties of titanium-oxo clusters (TOCs). However, the synthesis of such TOCs is highly challenging. In this work, we successfully synthesized a TBC4A-functionalized TOC, [Ti2(TBC4A)2(MeO)2] (Ti2; MeOH = methanol, TBC4A = tert-butylcalix[4]arene). By adjusting the solvent system, we successfully introduced 1,10-phenanthroline (Phen) and prepared TBC4A and Phen co-protected [Ti2(TBC4A)2(Phen)2] (Ti2-Phen). Moreover, when Phen was replaced with bulky 4,7-diphenyl-1,10-phenanthroline (Bphen), [Ti2(TBC4A)2(Bphen)2] (Ti2-Bphen), which is isostructural with Ti2-Phen, was obtained, demonstrating the generality of the synthetic method. Remarkably, Ti2-Phen demonstrates good stability and stronger light absorption, as well as superior photoelectric performance compared to Ti2. Density functional theory (DFT) calculations reveal that there exists ligand-to-core charge transfer (LCCT) in Ti2, while an unusual ligand-to-ligand charge transfer (LLCT) is present in Ti2-Phen, accompanied by partial LCCT. Therefore, the superior light absorption and photoelectric properties of Ti2-Phen are attributed to the existence of the unusual LLCT phenomenon. This study not only deeply explores the influence of Phen on the performance of the material but also provides a reference for the preparation of materials with excellent photoelectric performance.
Keywords: DFT calculations; calix[n]arenes; crystal structure; photoelectric performance; titanium-oxo clusters.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Auxiliary Carboxylate-Induced Assembly of Calix[6]arene-Polyoxotitanate Hybrid Systems with Photocatalytic Activity in the Oxidation of Sulfides.Inorg Chem. 2023 Apr 17;62(15):6047-6054. doi: 10.1021/acs.inorgchem.2c04528. Epub 2023 Apr 5. Inorg Chem. 2023. PMID: 37017204
-
All-catecholate-stabilized black titanium-oxo clusters for efficient photothermal conversion.Chem Sci. 2024 Jan 3;15(7):2655-2664. doi: 10.1039/d3sc05617a. eCollection 2024 Feb 14. Chem Sci. 2024. PMID: 38362423 Free PMC article.
-
Calixarene-Protected Titanium-Oxo Clusters and Their Photocurrent Responses and Photocatalytic Performances.Inorg Chem. 2021 Apr 5;60(7):5034-5041. doi: 10.1021/acs.inorgchem.1c00063. Epub 2021 Mar 8. Inorg Chem. 2021. PMID: 33677968
-
Topotactic Conversion of Titanium-Oxo Clusters to a Stable TOC-Based Metal-Organic Framework with the Selective Adsorption of Cationic Dyes.Inorg Chem. 2024 Apr 1;63(13):5961-5971. doi: 10.1021/acs.inorgchem.3c04608. Epub 2024 Mar 17. Inorg Chem. 2024. PMID: 38494631
-
Two titanium(iv)-oxo-clusters: synthesis, structures, characterization and recycling catalytic activity in the oxygenation of sulfides.Dalton Trans. 2017 Oct 24;46(41):14348-14355. doi: 10.1039/c7dt02516e. Dalton Trans. 2017. PMID: 29022617
References
-
- Chen R., Chen C.-L., Zhang H., Wang Z.-H., Sun F.-L., Du M.-H., Zhuang G.-L., Wang C., Long L.-S., Zheng L.-S., et al. Molecular solid solution of lanthanide-titanium-oxo clusters with enhanced photocatalytic hydrogen evolution. Sci. China Chem. 2024;67:529–535. doi: 10.1007/s11426-023-1847-x. - DOI
-
- Zhang Z., Han F., Fang J., Zhao C., Li S., Wu Y., Zhang Y., You S., Wu B., Li W. An Organic–Inorganic Hybrid Material Based on Benzo[ghi]perylenetri-imide and Cyclic Titanium-Oxo Cluster for Efficient Perovskite and Organic Solar Cells. CCS Chem. 2022;4:880–888. doi: 10.31635/ccschem.021.202100825. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

