Potential of Cell-Penetrating Peptide-Conjugated Antisense Oligonucleotides for the Treatment of SMA
- PMID: 38893532
- PMCID: PMC11173757
- DOI: 10.3390/molecules29112658
Potential of Cell-Penetrating Peptide-Conjugated Antisense Oligonucleotides for the Treatment of SMA
Abstract
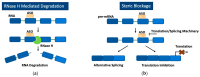
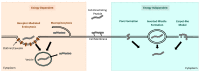
Spinal muscular atrophy (SMA) is a severe neuromuscular disorder that is caused by mutations in the survival motor neuron 1 (SMN1) gene, hindering the production of functional survival motor neuron (SMN) proteins. Antisense oligonucleotides (ASOs), a versatile DNA-like drug, are adept at binding to target RNA to prevent translation or promote alternative splicing. Nusinersen is an FDA-approved ASO for the treatment of SMA. It effectively promotes alternative splicing in pre-mRNA transcribed from the SMN2 gene, an analog of the SMN1 gene, to produce a greater amount of full-length SMN protein, to compensate for the loss of functional protein translated from SMN1. Despite its efficacy in ameliorating SMA symptoms, the cellular uptake of these ASOs is suboptimal, and their inability to penetrate the CNS necessitates invasive lumbar punctures. Cell-penetrating peptides (CPPs), which can be conjugated to ASOs, represent a promising approach to improve the efficiency of these treatments for SMA and have the potential to transverse the blood-brain barrier to circumvent the need for intrusive intrathecal injections and their associated adverse effects. This review provides a comprehensive analysis of ASO therapies, their application for the treatment of SMA, and the encouraging potential of CPPs as delivery systems to improve ASO uptake and overall efficiency.
Keywords: DG9; antisense oligonucleotides (ASOs); cell-penetrating peptides (CPPs); delivery; phosphorodiamidate morpholino oligomers (PMOs); spinal muscular atrophy (SMA).
Conflict of interest statement
T.Y. is a cofounder and shareholder of OligomicsTx Inc., which aims to commercialize antisense technology. Both authors declare that the research was conducted in absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

Similar articles
-
Systemic Injection of Antisense Oligos into Spinal Muscular Atrophy (SMA) Mice and Evaluation.Methods Mol Biol. 2025;2964:421-431. doi: 10.1007/978-1-0716-4730-1_27. Methods Mol Biol. 2025. PMID: 40720034
-
Challenges and future perspective of antisense therapy for spinal muscular atrophy: A review.Eur J Cell Biol. 2023 Jun;102(2):151326. doi: 10.1016/j.ejcb.2023.151326. Epub 2023 Jun 2. Eur J Cell Biol. 2023. PMID: 37295266 Review.
-
NCALD Antisense Oligonucleotide Therapy in Addition to Nusinersen further Ameliorates Spinal Muscular Atrophy in Mice.Am J Hum Genet. 2019 Jul 3;105(1):221-230. doi: 10.1016/j.ajhg.2019.05.008. Epub 2019 Jun 20. Am J Hum Genet. 2019. PMID: 31230718 Free PMC article.
-
Mechanistic principles of antisense targets for the treatment of spinal muscular atrophy.Future Med Chem. 2015;7(13):1793-808. doi: 10.4155/fmc.15.101. Epub 2015 Sep 18. Future Med Chem. 2015. PMID: 26381381 Free PMC article. Review.
-
In Vitro Evaluation of Antisense-Mediated Exon Inclusion for Spinal Muscular Atrophy.Methods Mol Biol. 2025;2964:405-420. doi: 10.1007/978-1-0716-4730-1_26. Methods Mol Biol. 2025. PMID: 40720033
Cited by
-
Recent Progress of Antisense Oligonucleotide Therapy for Superoxide-Dismutase-1-Mutated Amyotrophic Lateral Sclerosis: Focus on Tofersen.Genes (Basel). 2024 Oct 20;15(10):1342. doi: 10.3390/genes15101342. Genes (Basel). 2024. PMID: 39457466 Free PMC article. Review.
-
Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective.Int J Mol Sci. 2025 Jun 2;26(11):5350. doi: 10.3390/ijms26115350. Int J Mol Sci. 2025. PMID: 40508159 Free PMC article. Review.
References
-
- Prior T.W., Leach M.E., Finanger E. Spinal Muscular Atrophy. In: Adam M.P., Feldman J., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews(®) University of Washington; Seattle, WA, USA: 1993. - PubMed
-
- Hammond S.M., Hazell G., Shabanpoor F., Saleh A.F., Bowerman M., Sleigh J.N., Meijboom K.E., Zhou H., Muntoni F., Talbot K., et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 2016;113:10962–10967. doi: 10.1073/pnas.1605731113. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

