The Discovery of Selective Protein Arginine Methyltransferase 5 Inhibitors in the Management of β-Thalassemia through Computational Methods
- PMID: 38893537
- PMCID: PMC11173459
- DOI: 10.3390/molecules29112662
The Discovery of Selective Protein Arginine Methyltransferase 5 Inhibitors in the Management of β-Thalassemia through Computational Methods
Abstract
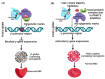
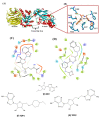
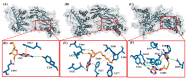
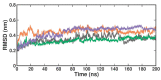
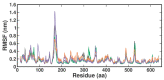
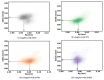
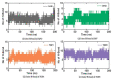
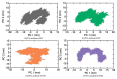
β-Thalassemia is an inherited genetic disorder associated with β-globin chain synthesis, which ultimately becomes anemia. Adenosine-2,3-dialdehyde, by inhibiting arginine methyl transferase 5 (PRMT5), can induce fetal hemoglobin (HbF) levels. Hence, the materialization of PRMT5 inhibitors is considered a promising therapy in the management of β-thalassemia. This study conducted a virtual screening of certain compounds similar to 5'-deoxy-5'methyladenosine (3XV) using the PubChem database. The top 10 compounds were chosen based on the best docking scores, while their interactions with the PRMT5 active site were analyzed. Further, the top two compounds demonstrating the lowest binding energy were subjected to drug-likeness analysis and pharmacokinetic property predictions, followed by molecular dynamics simulation studies. Based on the molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) score and molecular interactions, (3R,4S)-2-(6-aminopurin-9-yl)-5-[(4-ethylcyclohexyl)sulfanylmethyl]oxolane-3,4-diol (TOP1) and 2-(6-Aminopurin-9-yl)-5-[(6-aminopurin-9-yl)methylsulfanylmethyl]oxolane-3,4-diol (TOP2) were identified as potential hit compounds, while TOP1 exhibited higher binding affinity and stabler binding capabilities than TOP2 during molecular dynamics simulation (MDS) analysis. Taken together, the outcomes of our study could aid researchers in identifying promising PRMT5 inhibitors. Moreover, further investigations through in vivo and in vitro experiments would unquestionably confirm that this compound could be employed as a therapeutic drug in the management of β-thalassemia.
Keywords: PRMT5 inhibitors; dynamics; fetal hemoglobin; molecular docking; simulations; β-thalassemia.
Conflict of interest statement
The authors declare that they hold no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
- 2563-009/Presidential Ph.D. Scholarship, Chiang Mai University, Thailand
- PD2565/Post-Doctoral Fellowship 2022 (Reinventing University) through Dr. Yuvaraj Ravikumar, Chiang Mai University, Thailand
- BIO-2566-0574/CMU Faculty of Medicine Fund, Chiang Mai University, Thailand
- TGCMU2566P010/2566/Chiang Mai University, Thailand
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

