Graphene Oxide Strengthens Gelatine through Non-Covalent Interactions with Its Amorphous Region
- PMID: 38893573
- PMCID: PMC11173959
- DOI: 10.3390/molecules29112700
Graphene Oxide Strengthens Gelatine through Non-Covalent Interactions with Its Amorphous Region
Abstract
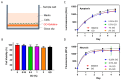
Graphene oxide (GO) has attracted huge attention in biomedical sciences due to its outstanding properties and potential applications. In this study, we synthesized GO using our recently developed 1-pyrenebutyric acid-assisted method and assessed how the GO as a filler influences the mechanical properties of GO-gelatine nanocomposite dry films as well as the cytotoxicity of HEK-293 cells grown on the GO-gelatine substrates. We show that the addition of GO (0-2%) improves the mechanical properties of gelatine in a concentration-dependent manner. The presence of 2 wt% GO increased the tensile strength, elasticity, ductility, and toughness of the gelatine films by about 3.1-, 2.5-, 2-, and 8-fold, respectively. Cell viability, apoptosis, and necrosis analyses showed no cytotoxicity from GO. Furthermore, we performed circular dichroism, X-ray diffraction, Fourier-transform infrared spectroscopy, and X-ray photoelectron spectroscopy analyses to decipher the interactions between GO and gelatine. The results show, for the first time, that GO enhances the mechanical properties of gelatine by forming non-covalent intermolecular interactions with gelatine at its amorphous or disordered regions. We believe that our findings will provide new insight and help pave the way for potential and wide applications of GO in tissue engineering and regenerative biomedicine.
Keywords: 2D nanomaterials; biomaterials; cytotoxicity; gelatine; graphene oxide; mechanical property of gelatine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

