Screening of Mpro Protease (SARS-CoV-2) Covalent Inhibitors from an Anthocyanin-Rich Blueberry Extract Using an HRMS-Based Analytical Platform
- PMID: 38893578
- PMCID: PMC11173886
- DOI: 10.3390/molecules29112702
Screening of Mpro Protease (SARS-CoV-2) Covalent Inhibitors from an Anthocyanin-Rich Blueberry Extract Using an HRMS-Based Analytical Platform
Abstract
Background: The viral main protease (Mpro) of SARS-CoV-2 has been recently proposed as a key target to inhibit virus replication in the host. Therefore, molecules that can bind the catalytic site of Mpro could be considered as potential drug candidates in the treatment of SARS-CoV-2 infections. Here we proposed the application of a state-of-the-art analytical platform which combines metabolomics and protein structure analysis to fish-out potential active compounds deriving from a natural matrix, i.e., a blueberry extract.
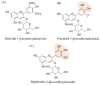
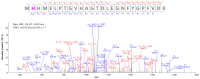
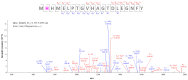
Methods: The experiments focus on finding MS covalent inhibitors of Mpro that contain in their structure a catechol/pyrogallol moiety capable of binding to the nucleophilic amino acids of the enzyme's catalytic site.
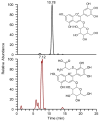
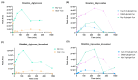
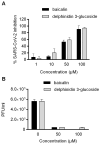
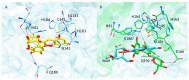
Results: Among the potential candidates identified, the delphinidin-3-glucoside showed the most promising results. Its antiviral activity has been confirmed in vitro on Vero E6 cells infected with SARS-CoV-2, showing a dose-dependent inhibitory effect almost comparable to the known Mpro inhibitor baicalin. The interaction of delphinidin-3-glucoside with the Mpro pocket observed was also evaluated by computational studies.
Conclusions: The HRMS analytical platform described proved to be effective in identifying compounds that covalently bind Mpro and are active in the inhibition of SARS-CoV-2 replication, such as delphinidin-3-glucoside.
Keywords: Mpro; SARS-CoV-2; blueberry; delphinidin-3-glucoside; mass spectrometry; metabolomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Investigating the promising SARS-CoV-2 main protease inhibitory activity of secoiridoids isolated from Jasminum humile; in silico and in Vitro assessments with structure-activity relationship.J Biomol Struct Dyn. 2024 Aug;42(13):6941-6953. doi: 10.1080/07391102.2023.2240419. Epub 2023 Jul 28. J Biomol Struct Dyn. 2024. PMID: 37505066
-
An integrated metabolomic and proteomic approach for the identification of covalent inhibitors of the main protease (Mpro) of SARS-COV-2 from crude natural extracts.Talanta. 2023 Jan 15;252:123824. doi: 10.1016/j.talanta.2022.123824. Epub 2022 Aug 12. Talanta. 2023. PMID: 36027618 Free PMC article.
-
Analysis of bioactive compounds of Olea europaea as potential inhibitors of SARS-CoV-2 main protease: a pharmacokinetics, molecular docking and molecular dynamics simulation studies.J Biomol Struct Dyn. 2025 Feb;43(3):1147-1158. doi: 10.1080/07391102.2023.2291172. Epub 2023 Dec 8. J Biomol Struct Dyn. 2025. PMID: 38063160
-
SARS-CoV-2 Mpro: A Potential Target for Peptidomimetics and Small-Molecule Inhibitors.Biomolecules. 2021 Apr 19;11(4):607. doi: 10.3390/biom11040607. Biomolecules. 2021. PMID: 33921886 Free PMC article. Review.
-
An Updated Review on SARS-CoV-2 Main Proteinase (MPro): Protein Structure and Small-Molecule Inhibitors.Curr Top Med Chem. 2021;21(6):442-460. doi: 10.2174/1568026620666201207095117. Curr Top Med Chem. 2021. PMID: 33292134 Review.
Cited by
-
Evaluating the Antiviral Potential of Polyherbal Formulation (Kabasura Kudineer) Against Monkeypox Virus: Targeting E5, Poxin, and DNA Polymerase Through Multifaceted Drug Discovery Approaches.Life (Basel). 2025 May 12;15(5):771. doi: 10.3390/life15050771. Life (Basel). 2025. PMID: 40430198 Free PMC article.
-
Exploring anti-SARS-CoV-2 natural products: dual-viral target inhibition by delphinidin and the anti-coronaviral efficacy of deapio platycodin D.Nat Prod Bioprospect. 2025 Jun 13;15(1):39. doi: 10.1007/s13659-025-00523-w. Nat Prod Bioprospect. 2025. PMID: 40512442 Free PMC article.
References
-
- [(accessed on 26 May 2023)]. Available online: https://cov-lineages.org/lineage_list.html.
-
- Sacco M.D., Ma C., Lagarias P., Gao A., Townsend J.A., Meng X., Dube P., Zhang X., Hu Y., Kitamura N., et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv. 2020;6:eabe0751. doi: 10.1126/sciadv.abe0751. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

