Nationwide Real-World Data of Microsatellite Instability and/or Mismatch Repair Deficiency in Cancer: Prevalence and Testing Patterns
- PMID: 38893603
- PMCID: PMC11171982
- DOI: 10.3390/diagnostics14111076
Nationwide Real-World Data of Microsatellite Instability and/or Mismatch Repair Deficiency in Cancer: Prevalence and Testing Patterns
Abstract
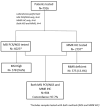
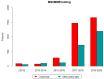
Determination of microsatellite instability (MSI)/mismatch repair (MMR) status in cancer has several clinical implications. Our aim was to integrate MSI/MMR status from patients tested in Greece to assess the prevalence of MSI-high (MSI-H)/deficient MMR (dMMR) per tumor type, testing patterns over time and concordance between MSI and MMR status. We retrospectively recorded MSI/MMR testing data of patients with diverse tumor types performed in pathology and molecular diagnostics laboratories across Greece. Overall, 18 of 22 pathology and/or molecular diagnostics laboratories accepted our invitation to participate. In the 18 laboratories located across the country, 7916 tumor samples were evaluated for MSI/MMR status. MSI/MMR testing significantly increased in patients with colorectal cancer (CRC) and other tumor types overtime (p < 0.05). The highest prevalence was reported in endometrial cancer (47 of 225 patients, 20.9%). MSI-H/dMMR was observed in most tumor types, even in low proportions. Among 904 tumors assessed both for MSI and MMR status, 21 had discordant results (overall discordance rate, 2.3%). We reported MSI-H/dMMR prevalence rates in patients with diverse cancers, while demonstrating increasing referral patterns from medical oncologists in the country overtime. The anticipated high rate of concordance between MSI and MMR status in paired analysis was confirmed.
Keywords: Greece; MMR; MSI; biomarker; immunotherapy; nationwide; real-world data.
Conflict of interest statement
EF declares speaker fees from Roche, AstraZeneca, GSK, Amgen and Pfizer, travel grants from AstraZeneca and Genesis, participation on advisory board of Amgen and stock ownership in Deciphera Pharmaceuticals, Inc. and Genprex. GF declares honoraria from AstraZeneca and Novartis, participation on advisory board of Pfizer and Novartis and stock ownership in Genprex, Daiichi Sankyo, RFL Holdings and FORMYCON. TP, GT are KARYO employees; EP, GN are Genekor M.S.A. employees; CG, SM are BioPath Innovations S.A. employees; PC, GC are Genotypos Science Labs M.S.A. employees; DH, EMM are microDiagnostics LP employees; EE is Istodierevnitiki S.A. employee.
Figures
References
-
- Zaanan A., Shi Q., Taieb J., Alberts S.R., Meyers J.P., Smyrk T.C., Julie C., Zawadi A., Tabernero J., Mini E., et al. Role of Deficient DNA Mismatch Repair Status in Patients With Stage III Colon Cancer Treated With FOLFOX Adjuvant Chemotherapy: A Pooled Analysis from 2 Randomized Clinical Trials. JAMA Oncol. 2018;4:379–383. doi: 10.1001/jamaoncol.2017.2899. - DOI - PMC - PubMed
-
- Sinicrope F.A., Mahoney M.R., Smyrk T.C., Thibodeau S.N., Warren R.S., Bertagnolli M.M., Nelson G.D., Goldberg R.M., Sargent D.J., Alberts S.R. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:3664–3672. doi: 10.1200/jco.2013.48.9591. - DOI - PMC - PubMed
-
- Sargent D.J., Marsoni S., Monges G., Thibodeau S.N., Labianca R., Hamilton S.R., French A.J., Kabat B., Foster N.R., Torri V., et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:3219–3226. doi: 10.1200/jco.2009.27.1825. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources